The Correlation between Physical Crosslinking and Water-Soluble Drug Release from Chitosan-Based Microparticles
- PMID: 32429349
- PMCID: PMC7284795
- DOI: 10.3390/pharmaceutics12050455
The Correlation between Physical Crosslinking and Water-Soluble Drug Release from Chitosan-Based Microparticles
Abstract
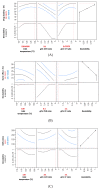
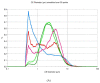
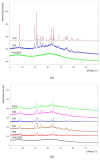
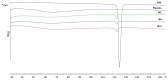
Microparticles containing water-soluble zidovudine were prepared by spray-drying using chitosan glutamate and beta-glycerophosphate as an ion crosslinker (CF). The Box-Behnken design was applied to optimize the microparticles in terms of their drug loading and release behavior. Physicochemical studies were undertaken to support the results from dissolution tests and to evaluate the impact of the crosslinking ratio on the microparticles' characteristics. The zidovudine dissolution behavior had a complex nature which comprised two phases: an initial burst effect followed with a prolonged release stage. The initial drug release, which can be modulated by the crosslinking degree, was primarily governed by the dissolution of the drug crystals located on the microparticles' surfaces. In turn, the further dissolution stage was related to the drug diffusion from the swollen polymer matrix and was found to correlate with the drug loading. Differential Scanning Calorimetry (DSC) studies revealed the partial incorporation of a non-crystallized drug within the polymer matrix, which correlated with the amount of CF. Although CF influenced the swelling capacity of chitosan glutamate microparticles, surprisingly a higher amount of CF did not impact the time required for 80% of the drug to be released markedly. The formulation with the lowest polymer:CF ratio, 3:1, was selected as optimal, providing satisfactory drug loading and displaying a moderate burst effect within the first 30 min of the study, followed with a prolonged drug release of up to 210 min.
Keywords: chitosan glutamate; drug release; ion crosslinker; microparticles; zidovudine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Romić M.D., Klarić M.Š., Lovrić J., Pepić I., Cetina-Čižmek B., Filipović-Grčić J., Hafner A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016;107:67–79. doi: 10.1016/j.ejpb.2016.06.013. - DOI - PubMed
-
- Lisuzzo L., Cavallaro G., Parisi F., Milioto S., Fakhrullin R., Lazzara G. Core/shell gel beads with embedded halloysite nanotubes for controlled drug release. Coatings. 2019;9:70. doi: 10.3390/coatings9020070. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

