Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study
- PMID: 32429365
- PMCID: PMC7281323
- DOI: 10.3390/cancers12051256
Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study
Abstract
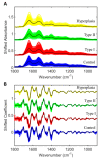
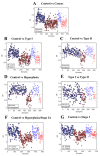
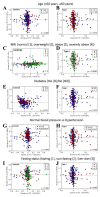
Endometrial cancer is the sixth most common cancer in women, with a rising incidence worldwide. Current approaches for the diagnosis and screening of endometrial cancer are invasive, expensive or of moderate diagnostic accuracy, limiting their clinical utility. There is a need for cost-effective and minimally invasive approaches to facilitate the early detection and timely management of endometrial cancer. We analysed blood plasma samples in a cross-sectional diagnostic accuracy study of women with endometrial cancer (n = 342), its precursor lesion atypical hyperplasia (n = 68) and healthy controls (n = 242, total n = 652) using attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopy and machine learning algorithms. We show that blood-based infrared spectroscopy has the potential to detect endometrial cancer with 87% sensitivity and 78% specificity. Its accuracy is highest for Type I endometrial cancer, the most common subtype, and for atypical hyperplasia, with sensitivities of 91% and 100%, and specificities of 81% and 88%, respectively. Our large-cohort study shows that a simple blood test could enable the early detection of endometrial cancer of all stages in symptomatic women and provide the basis of a screening tool in high-risk groups. Such a test has the potential not only to differentially diagnose endometrial cancer but also to detect its precursor lesion atypical hyperplasia-the early recognition of which may allow fertility sparing management and cancer prevention.
Keywords: blood diagnostics; endometrial cancer; screening; spectroscopy.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
-
- Cancer Research, UK. [(accessed on 12 May 2020)]; Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s....
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

