Exogenous Liposomal Ceramide-C6 Ameliorates Lipidomic Profile, Energy Homeostasis, and Anti-Oxidant Systems in NASH
- PMID: 32429478
- PMCID: PMC7290333
- DOI: 10.3390/cells9051237
Exogenous Liposomal Ceramide-C6 Ameliorates Lipidomic Profile, Energy Homeostasis, and Anti-Oxidant Systems in NASH
Abstract
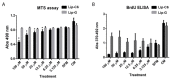
In non-alcoholic steatohepatitis (NASH), many lines of investigation have reported a dysregulation in lipid homeostasis, leading to intrahepatic lipid accumulation. Recently, the role of dysfunctional sphingolipid metabolism has also been proposed. Human and animal models of NASH have been associated with elevated levels of long chain ceramides and pro-apoptotic sphingolipid metabolites, implicated in regulating fatty acid oxidation and inflammation. Importantly, inhibition of de novo ceramide biosynthesis or knock-down of ceramide synthases reverse some of the pathology of NASH. In contrast, cell permeable, short chain ceramides have shown anti-inflammatory actions in multiple models of inflammatory disease. Here, we investigated non-apoptotic doses of a liposome containing short chain C6-Ceramide (Lip-C6) administered to human hepatic stellate cells (hHSC), a key effector of hepatic fibrogenesis, and an animal model characterized by inflammation and elevated liver fat content. On the basis of the results from unbiased liver transcriptomic studies from non-alcoholic fatty liver disease patients, we chose to focus on adenosine monophosphate activated kinase (AMPK) and nuclear factor-erythroid 2-related factor (Nrf2) signaling pathways, which showed an abnormal profile. Lip-C6 administration inhibited hHSC proliferation while improving anti-oxidant protection and energy homeostasis, as indicated by upregulation of Nrf2, activation of AMPK and an increase in ATP. To confirm these in vitro data, we investigated the effect of a single tail-vein injection of Lip-C6 in the methionine-choline deficient (MCD) diet mouse model. Lip-C6, but not control liposomes, upregulated phospho-AMPK, without inducing liver toxicity, apoptosis, or exacerbating inflammatory signaling pathways. Alluding to mechanism, mass spectrometry lipidomics showed that Lip-C6-treatment reversed the imbalance in hepatic phosphatidylcholines and diacylglycerides species induced by the MCD-fed diet. These results reveal that short-term Lip-C6 administration reverses energy/metabolic depletion and increases protective anti-oxidant signaling pathways, possibly by restoring homeostatic lipid function in a model of liver inflammation with fat accumulation.
Keywords: adenosine monophosphate-activated kinase (AMPK); apoptosis; ceramides; diacylglycerol (DG); human hepatic stellate cells (hHSC); inflammation; lipidomics; liposomes; methionine-choline deficient diet (MCD); non-alcoholic steatohepatitis (NASH); nuclear factor-erythroid 2-related factor 2 (Nfe2l2/NRF2); phosphatidylcholine (PC).
Conflict of interest statement
“The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”. Penn State Research Foundation has licensed ceramide nanotechnology to Keystone Nano, Inc. (PA, USA) and M.K. is cofounder and Chief Medical Officer of Keystone Nano. R.B. has done consulting services for Verlyx Pharma Inc. G.M., L.F., and L.L. are now full time employees at Engitix Ltd., and G.M., L.L., M.P., and K.R. own shares in Engitix Ltd. M.P. and K.R. receive consultancies from Engitix Ltd.
Figures