Functional Characterization of Neurofilament Light Splicing and Misbalance in Zebrafish
- PMID: 32429483
- PMCID: PMC7291018
- DOI: 10.3390/cells9051238
Functional Characterization of Neurofilament Light Splicing and Misbalance in Zebrafish
Abstract
Neurofilaments (NFs), a major cytoskeletal component of motor neurons, play a key role in the differentiation, establishment and maintenance of their morphology and mechanical strength. The de novo assembly of these neuronal intermediate filaments requires the presence of the neurofilament light subunit (NEFL), whose expression is reduced in motor neurons in amyotrophic lateral sclerosis (ALS). This study used zebrafish as a model to characterize the NEFL homologue neflb, which encodes two different isoforms via a splicing of the primary transcript (neflbE4 and neflbE3). In vivo imaging showed that neflb is crucial for proper neuronal development, and that disrupting the balance between its two isoforms specifically affects the NF assembly and motor axon growth, with resultant motor deficits. This equilibrium is also disrupted upon the partial depletion of TDP-43 (TAR DNA-binding protein 43), an RNA-binding protein encoded by the gene TARDBP that is mislocalized into cytoplasmic inclusions in ALS. The study supports the interaction of the NEFL expression and splicing with TDP-43 in a common pathway, both biologically and pathogenetically.
Keywords: amyotrophic lateral sclerosis (ALS), neurofilament light (NEFL), TDP-43; neurofilaments (NFs); zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

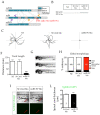
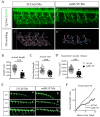
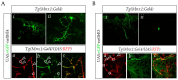
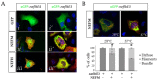

References
-
- Scott D., Smith K.E., O’Brien B.J., Angelides K.J. Characterization of mammalian neurofilament triplet proteins. Subunit stoichiometry and morphology of native and reconstituted filaments. J. Biol. Chem. 1985;260:10736–10747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

