Multi-Stress Induction of the Mycobacterium tuberculosis MbcTA Bactericidal Toxin-Antitoxin System
- PMID: 32429486
- PMCID: PMC7291234
- DOI: 10.3390/toxins12050329
Multi-Stress Induction of the Mycobacterium tuberculosis MbcTA Bactericidal Toxin-Antitoxin System
Abstract
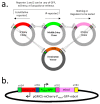
MbcTA is a type II toxin/antitoxin (TA) system of Mycobacterium tuberculosis. The MbcT toxin triggers mycobacterial cell death in vitro and in vivo through the phosphorolysis of the essential metabolite NAD+ and its bactericidal activity is neutralized by physical interaction with its cognate antitoxin MbcA. Therefore, the MbcTA system appears as a promising target for the development of novel therapies against tuberculosis, through the identification of compounds able to antagonize or destabilize the MbcA antitoxin. Here, the expression of the mbcAT operon and its regulation were investigated. A dual fluorescent reporter system was developed, based on an integrative mycobacterial plasmid that encodes a constitutively expressed reporter, serving as an internal standard for monitoring mycobacterial gene expression, and an additional reporter, dependent on the promoter under investigation. This system was used both in M. tuberculosis and in the fast growing model species Mycobacterium smegmatis to: (i) assess the autoregulation of mbcAT; (ii) perform a genetic dissection of the mbcA promoter/operator region; and (iii) explore the regulation of mbcAT transcription from the mbcA promoter (PmbcA) in a variety of stress conditions, including in vivo in mice and in macrophages.
Keywords: NAD+; bacterial cell death; stress-response; toxin-antitoxin systems; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Freire D.M., Gutierrez C., Garza-Garcia A., Grabowska A.D., Sala A.J., Ariyachaokun K., Panikova T., Beckham K.S.H., Colom A., Pogenberg V., et al. An NAD(+) Phosphorylase Toxin Triggers Mycobacterium tuberculosis Cell Death. Mol. Cell. 2019;73:1282–1291.e8. doi: 10.1016/j.molcel.2019.01.028. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

