Chemical and Biological Aspects of Montanine-Type Alkaloids Isolated from Plants of the Amaryllidaceae Family
- PMID: 32429491
- PMCID: PMC7288066
- DOI: 10.3390/molecules25102337
Chemical and Biological Aspects of Montanine-Type Alkaloids Isolated from Plants of the Amaryllidaceae Family
Abstract
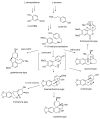
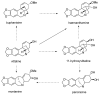
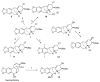
Plants of the Amaryllidaceae family are promising therapeutic tools for human diseases and have been used as alternative medicines. The specific secondary metabolites of this plant family, called Amaryllidaceae alkaloids (AA), have attracted considerable attention due to their interesting pharmacological activities. One of them, galantamine, is already used in the therapy of Alzheimer's disease as a long acting, selective, reversible inhibitor of acetylcholinesterase. One group of AA is the montanine-type, such as montanine, pancracine and others, which share a 5,11-methanomorphanthridine core. So far, only 14 montanine-type alkaloids have been isolated. Compared with other structural-types of AA, montanine-type alkaloids are predominantly present in plants in low concentrations, but some of them display promising biological properties, especially in vitro cytotoxic activity against different cancerous cell lines. The present review aims to summarize comprehensively the research that has been published on the Amaryllidaceae alkaloids of montanine-type.
Keywords: Amaryllidaceae; alkaloids; biological activity; derivatives; montanine; montanine-type; pancracine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Dahlgren R.M.T., Clifford H.T., Yeo P.F. The Families of the Monocotyledons. Structure, Evolution and Taxonomy. 1st ed. Springer; New York, NY, USA: 1985. pp. 1–520.
-
- Elgorashi E.E., van Staden J. Bioactivity and bioactive compounds from African Amaryllidaceae. In: Juliani H.R., Simon J.E., Ho C.T., editors. African Natural Plant Products: New Discoveries and Challenges in Chemistry and Quality. Volume 1021. American Chemical Society; Washington, DC, USA: 2009. pp. 151–170. (ACS Symposium Series).
-
- Dalecká M., Havelek R., Královec K., Brůčková L., Cahlíková L. Amaryllidaceae family alkaloids as potential drugs for cancer treatment. Chem. Listy. 2013;107:701–708.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

