Complementary Targeting of Rb Phosphorylation and Growth in Cervical Cancer Cell Cultures and a Xenograft Mouse Model by SHetA2 and Palbociclib
- PMID: 32429557
- PMCID: PMC7281234
- DOI: 10.3390/cancers12051269
Complementary Targeting of Rb Phosphorylation and Growth in Cervical Cancer Cell Cultures and a Xenograft Mouse Model by SHetA2 and Palbociclib
Abstract
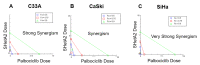
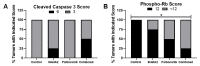
Cervical cancer is caused by high-risk human papillomavirus (HPV) types and treated with conventional chemotherapy with surgery and/or radiation. HPV E6 and E7 proteins increase phosphorylation of retinoblastoma (Rb) by cyclin D1/cyclin dependent kinase (CDK)4/6 complexes. We hypothesized that cyclin D1 degradation by the SHetA2 drug in combination with palbociclib inhibition of CDK4/6 activity synergistically reduces phosphorylated Rb (phospho-Rb) and inhibits cervical cancer growth. The effects of these drugs, alone, and in combination, were evaluated in SiHa and CaSki HPV-positive and C33A HPV-negative cervical cancer cell lines using cell culture, western blots and ELISA, and in a SiHa xenograft model. Endpoints were compared by isobolograms, ANOVA, and Chi-Square. In all cell lines, combination indexes documented synergistic interaction of SHetA2 and palbociclib in association SHetA2 reduction of cyclin D1 and phospho-Rb, palbociclib reduction of phospho-Rb, and enhanced phospho-Rb reduction upon drug combination. Both drugs significantly reduced phospho-Rb and growth of SiHa xenograft tumors as single agents and acted additively when combined, with no evidence of toxicity. Dilated CD31-negative blood vessels adjacent to, or within, areas of necrosis and apoptosis were observed in all drug-treated tumors. These results justify development of the SHetA2 and palbociclib combination for targeting phospho-Rb in cervical cancer treatment.
Keywords: SHetA2; cervical cancer; cyclin D1; palbociblib; retinoblastoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Manipulation of metabolic responses enhances SHetA2 efficacy without toxicity in cervical cancer cell lines and xenografts.Gynecol Oncol. 2024 Jan;180:44-54. doi: 10.1016/j.ygyno.2023.11.013. Epub 2023 Dec 5. Gynecol Oncol. 2024. PMID: 38052108 Free PMC article.
-
Targeting Cyclin-Dependent Kinases in Synovial Sarcoma: Palbociclib as a Potential Treatment for Synovial Sarcoma Patients.Ann Surg Oncol. 2016 Sep;23(9):2745-52. doi: 10.1245/s10434-016-5341-x. Epub 2016 Jun 22. Ann Surg Oncol. 2016. PMID: 27334220 Free PMC article.
-
RAF inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6 inhibition by abemaciclib via superior inhibition of phospho-RB and suppression of cyclin D1.Oncogene. 2018 Feb 8;37(6):821-832. doi: 10.1038/onc.2017.384. Epub 2017 Oct 23. Oncogene. 2018. PMID: 29059158
-
Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs.Pharmacol Res. 2016 May;107:249-275. doi: 10.1016/j.phrs.2016.03.012. Epub 2016 Mar 16. Pharmacol Res. 2016. PMID: 26995305 Review.
-
Targeting breast cancer with CDK inhibitors.Curr Oncol Rep. 2015;17(5):443. doi: 10.1007/s11912-015-0443-3. Curr Oncol Rep. 2015. PMID: 25716100 Review.
Cited by
-
Pharmacodynamics of Cyclin D1 Degradation in Ovarian Cancer Xenografts with Repeated Oral SHetA2 Dosing.AAPS J. 2023 Dec 12;26(1):5. doi: 10.1208/s12248-023-00874-7. AAPS J. 2023. PMID: 38087107 Free PMC article.
-
Utility and Mechanism of SHetA2 and Paclitaxel for Treatment of Endometrial Cancer.Cancers (Basel). 2021 May 12;13(10):2322. doi: 10.3390/cancers13102322. Cancers (Basel). 2021. PMID: 34066052 Free PMC article.
-
Targeting HSP70-E7 Interaction With SHetA2: A Novel Therapeutic Strategy for Cervical Cancer.J Med Virol. 2024 Nov;96(11):e70088. doi: 10.1002/jmv.70088. J Med Virol. 2024. PMID: 39588793
-
SHetA2 Attack on Mortalin and Colleagues in Cancer Therapy and Prevention.Front Cell Dev Biol. 2022 Feb 23;10:848682. doi: 10.3389/fcell.2022.848682. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35281109 Free PMC article. Review.
-
Distinct mechanism of cervical cancer cell death caused by the investigational new drug SHetA2.Front Oncol. 2022 Sep 20;12:958536. doi: 10.3389/fonc.2022.958536. eCollection 2022. Front Oncol. 2022. PMID: 36203464 Free PMC article.
References
-
- Cibula D., Potter R., Planchamp F., Avall-Lundqvist E., Fischerova D., Haie Meder C., Kohler C., Landoni F., Lax S., Lindegaard J.C., et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018;127:404–416. doi: 10.1016/j.radonc.2018.03.003. - DOI - PubMed
-
- NCC Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Cervical Cancer. [(accessed on 2 December 2019)]; Available online: https://www.nccn.org/
-
- Tewari K.S., Sill M.W., Penson R.T., Huang H., Ramondetta L.M., Landrum L.M., Oaknin A., Reid T.J., Leitao M.M., Michael H.E., et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. - DOI - PMC - PubMed
-
- Society A.C. Cancer Facts & Figures 2019. American Cancer Society; Atlanta, GA, USA: 2019.
-
- Senkomago V., Duran D., Loharikar A., Hyde T.B., Markowitz L.E., Unger E.R., Saraiya M. CDC Activities for Improving Implementation of Human Papillomavirus Vaccination, Cervical Cancer Screening, and Surveillance Worldwide. Emerg. Infect. Dis. 2017;23:101. doi: 10.3201/eid2313.170603. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

