Loss of homeostatic microglial phenotype in CSF1R-related Leukoencephalopathy
- PMID: 32430064
- PMCID: PMC7236286
- DOI: 10.1186/s40478-020-00947-0
Loss of homeostatic microglial phenotype in CSF1R-related Leukoencephalopathy
Erratum in
-
Correction to: Loss of homeostatic microglial phenotype in CSF1R-related Leukoencephalopathy.Acta Neuropathol Commun. 2020 Jun 24;8(1):90. doi: 10.1186/s40478-020-00970-1. Acta Neuropathol Commun. 2020. PMID: 32580749 Free PMC article.
Abstract
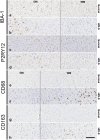
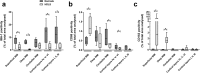
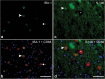
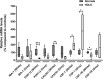
Microglia are resident macrophages of the central nervous system, and their unique molecular signature is dependent upon CSF-1 signaling. Previous studies have demonstrated the importance of CSF-1R in survival and development of microglia in animal models, but the findings are of uncertain relevance to understanding the influence of CSF-1R on microglia in humans. Hereditary diffuse leukoencephalopathy with spheroids (HDLS) [also known as adult onset leukoencephalopathy with spheroids and pigmented glia (ALSP)] is a neurodegenerative disorder primarily affecting cerebral white matter, most often caused by mutations of CSF1R. Therefore, we hypothesized that the molecular profile of microglia may be affected in HDLS. Semi-quantitative immunohistochemistry and quantitative transcriptomic profiling revealed reduced expression of IBA-1 and P2RY12 in both white and gray matter microglia of HDLS. In contrast, there was increased expression of CD68 and CD163 in microglia in affected white matter. In addition, expression of selective and specific microglial markers, including P2RY12, CX3CR1 and CSF-1R, were reduced in affected white matter. These results suggest that microglia in white matter in HDLS lose their homeostatic phenotype. Supported by gene ontology analysis, it is likely that an inflammatory phenotype is a key pathogenic feature of microglia in vulnerable brain regions of HDLS. Our findings suggest a potential mechanism of disease pathogenesis by linking aberrant CSF-1 signaling to altered microglial phenotype. They also support the idea that HDLS may be a primary microgliopathy. We observed increased expression of CSF-2 in gray matter compared to affected white matter, which may contribute to selective vulnerability of white matter in HDLS. Our findings suggest that methods that restore the homeostatic phenotype of microglia might be considered treatment approaches in HDLS.
Keywords: Adult leukoencephalopathy with spheroids and pigmented glia (ALSP); CSF-1; CSF-1R; CSF-1R-related leukoencephalopathy; Hereditary diffuse leukoencephalopathy with spheroids (HDLS); Immunohistochemistry; Microglia; RNA expression profiling.
Conflict of interest statement
The authors declare that they have no conflicts of interest with respect to the contents of this manuscript.
Figures


References
-
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55:687–700. - PubMed
-
- Axelsson R, Röyttä M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl. 1984;314:1–65. - PubMed
-
- Baba Y, Ghetti B, Baker MC, Uitti RJ, Hutton ML, Yamaguchi K, Bird T, Lin W, DeLucia MW, Dickson DW, Wszolek ZK. Hereditary diffuse leukoencephalopathy with spheroids: clinical, pathologic and genetic studies of a new kindred. Acta Neuropathol. 2006;111:300–311. - PubMed
-
- Borda JT, Alvarez X, Mohan M, Hasegawa A, Bernardino A, Jean S, Aye P, Lackner AA. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol. 2008;172:725–737. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- P30 AG066444/AG/NIA NIH HHS/United States
- R01 AG053500/AG/NIA NIH HHS/United States
- R21 NS101673/NS/NINDS NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- R01 EY027921/EY/NEI NIH HHS/United States
- UH3 NS103870/NS/NINDS NIH HHS/United States
- R01 AG054102/AG/NIA NIH HHS/United States
- P01 NS084974/NS/NINDS NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- UG3 NS103870/NS/NINDS NIH HHS/United States
- R01 AG051812/AG/NIA NIH HHS/United States
- R01 NS088137/NS/NINDS NIH HHS/United States
- R01 AG054449/AG/NIA NIH HHS/United States
- R35 NS097261/NS/NINDS NIH HHS/United States
- R01 AG053242/AG/NIA NIH HHS/United States
- R21 AG050804/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- RF1 AG051504/AG/NIA NIH HHS/United States
- R01 AG054672/AG/NIA NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- U01 AG045390/AG/NIA NIH HHS/United States
- UG3 NS104095/NS/NINDS NIH HHS/United States
- RF1 AG057181/AG/NIA NIH HHS/United States
- U54 NS100693/NS/NINDS NIH HHS/United States
- R21 NS104609/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

