The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies
- PMID: 32430651
- PMCID: PMC7236433
- DOI: 10.1007/s11739-020-02364-6
The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies
Abstract
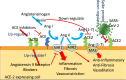
SARS-CoV-2 is characterized by a spike protein allowing viral binding to the angiotensin-converting enzyme (ACE)-2, which acts as a viral receptor and is expressed on the surface of several pulmonary and extra-pulmonary cell types, including cardiac, renal, intestinal and endothelial cells. There is evidence that also endothelial cells are infected by SARS-COV-2, with subsequent occurrence of systemic vasculitis, thromboembolism and disseminated intravascular coagulation. Those effects, together with the "cytokine storm" are involved in a worse prognosis. In clinical practice, angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin II receptor blockers (ARBs) are extensively used for the treatment of hypertension and other cardiovascular diseases. In in vivo studies, ACE-Is and ARBs seem to paradoxically increase ACE-2 expression, which could favour SARS-CoV-2 infection of host's cells and tissues. By contrast, in patients treated with ACE-Is and ARBs, ACE-2 shows a downregulation at the mRNA and protein levels in kidney and cardiac tissues. Yet, it has been claimed that both ARBs and ACE-Is could result potentially useful in the clinical course of SARS-CoV-2-infected patients. As detected in China and as the Italian epidemiological situation confirms, the most prevalent comorbidities in deceased patients with COVID-19 are hypertension, diabetes and cardiovascular diseases. Older COVID-19-affected patients with cardiovascular comorbidities exhibit a more severe clinical course and a worse prognosis, with many of them being also treated with ARBs or ACE-Is. Another confounding factor is cigarette smoking, which has been reported to increase ACE-2 expression in both experimental models and humans. Sex also plays a role, with chromosome X harbouring the gene coding for ACE-2, which is one of the possible explanations of why mortality in female patients is lower. Viral entry also depends on TMPRSS2 protease activity, an androgen dependent enzyme. Despite the relevance of experimental animal studies, to comprehensively address the question of the potential hazards or benefits of ACE-Is and ARBs on the clinical course of COVID-19-affected patients treated by these anti-hypertensive drugs, we will need randomized human studies. We claim the need of adequately powered, prospective studies aimed at answering the following questions of paramount importance for cardiovascular, internal and emergency medicine: Do ACE-Is and ARBs exert similar or different effects on infection or disease course? Are such effects dangerous, neutral or even useful in older, COVID-19-affected patients? Do they act on multiple cell types? Since ACE-Is and ARBs have different molecular targets, the clinical course of SARS-CoV-2 infection could be also different in patients treated by one or the other of these two drug classes. At present, insufficient detailed data from trials have been made available.
Keywords: ACE-inhibitors; Angiotensin II receptor blockers; COVID-19; Cardiovascular disease; Endothelium; Thrombosis.
Conflict of interest statement
The authors declare that this “Point of view” article was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Comment in
-
The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies: comment.Intern Emerg Med. 2020 Nov;15(8):1581-1582. doi: 10.1007/s11739-020-02406-z. Epub 2020 Jun 20. Intern Emerg Med. 2020. PMID: 32564289 Free PMC article. No abstract available.
References
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous