Effect of treatment with a full-occlusion biofeedback splint on sleep bruxism and TMD pain: a randomized controlled clinical trial
- PMID: 32430774
- PMCID: PMC7544753
- DOI: 10.1007/s00784-020-03270-z
Effect of treatment with a full-occlusion biofeedback splint on sleep bruxism and TMD pain: a randomized controlled clinical trial
Abstract
Objectives: The purpose of the present study was to analyze treatment outcome with a full-occlusion biofeedback (BFB) splint on sleep bruxism (SB) and TMD pain compared with treatment with an adjusted occlusal splint (AOS).
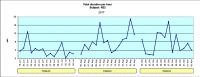
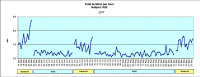
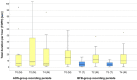
Materials and methods: Forty-one patients were randomly allocated to a test (BFB) or a control (AOS) group and monitored over a 3-month period. Output variables were frequency and duration of bruxing events (bursts) and various pain symptoms.
Results: The BFB group showed a statistically significant reduction in the frequency and duration of bursts and a statistically significant improvement in the patients' global well-being and the facial muscle pain parameter. After the treatment was stopped, the BFB group showed a statistically significant reduction in the average and maximum duration but no statistically significant change in the frequency of bursts.
Conclusions: The tested BFB splint is highly effective in reducing SB at the subconscious level, i.e., without waking the patient, and in achieving improvements in global pain perception. The results suggest that the BFB splint also provides a better treatment option for bruxism-related pain than an AOS. However, further research is needed, and specifically studies with a larger patient population displaying higher levels of pain at baseline.
Clinical relevance: By reducing burst duration and therefore the pathological load on the masticatory apparatus, the BFB splint reduces TMD and bruxism-related symptoms and improves patients' physical well-being. In the long term, this could prevent damage to the TMJ. This study confirms the effectiveness and safety of this splint.
The universal trial number: U1111-1239-2450 DRKS-ID REGISTRATION: DRKS00018092.
Keywords: Biofeedback/therapy; Occlusal splint; Sleep bruxism/therapy; Temporomandibular disorders; Vibration.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Comment in
-
MORE EVIDENCE IS NEEDED TO DETERMINE THE EFFECTIVENESS OF FULL OCCLUSION BIOFEEDBACK SPLINTS USED FOR SLEEP BRUXISM (SB) AND TEMPOROMANDIBULAR DISORDER (TMD) PAIN.J Evid Based Dent Pract. 2021 Dec;21(4):101650. doi: 10.1016/j.jebdp.2021.101650. Epub 2021 Oct 24. J Evid Based Dent Pract. 2021. PMID: 34922723
References
-
- Deutsche Gesellschaft für Funktionsdiagnostik und -therapie in der Zahn- M-uKD, Deutsche Gesellschaft für Zahn- M-uKD (2019) S3-Leitlinie (Langversion) Diagnostik und Behandlung von Bruxismus. AWMForg https://wwwawmforg/leitlinien/detail/ll/083-027html Accessed January 6, 2020 2020
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

