Noninvasive Models for Predicting Liver Fibrosis in Individuals with Hepatitis D Virus/Hepatitis B Virus Coinfection in the Brazilian Amazon Region
- PMID: 32431268
- PMCID: PMC7356423
- DOI: 10.4269/ajtmh.19-0688
Noninvasive Models for Predicting Liver Fibrosis in Individuals with Hepatitis D Virus/Hepatitis B Virus Coinfection in the Brazilian Amazon Region
Abstract
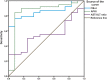
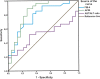
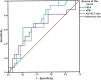
Hepatitis D virus (HDV) genotype III is endemic in the western Amazon basin and is considered to cause the most severe form of chronic viral hepatitis. Recently, noninvasive fibrosis scores to determine the stage of liver fibrosis have been evaluated in individuals positive for HDV genotype I, but their utility in HDV genotype III-positive patients is unknown. In this retrospective study conducted in an outpatient viral hepatitis referral clinic in the Brazilian Amazon region, the aspartate aminotransferase (AST) to Aspartate aminotransferase to Platelet Ratio Index (APRI) and Fibrosis Index for Liver Fibrosis (FIB-4) values were calculated and compared with histological fibrosis stages. Among the 50 patients analyzed, the median age at liver biopsy was 35.6 years, 66% were male, and all had compensated liver disease. Histological staging revealed fibrosis stages 0, 1, 2, 3, and 4 in four (8%), eight (16%), 11 (22), 11 (22%), and 16 (32%) patients, respectively. The area under the receiver operating curve (AUROC) of AST-to-alanine aminotransferase (ALT) ratio, APRI, and FIB-4 for detection of significant fibrosis (F ≥ 2) was 0.550 (P = 0.601), 0.853 (P < 0.001), and 0.853 (P < 0.0001), respectively. Lower AUROC values were obtained for cirrhosis: the AST-to-ALT ratio was 0.640 (P = 0.114), APRI was 0.671 (P = 0.053), and FIB-4 was 0.701 (P = 0.023). The optimal cutoff value for significant fibrosis for APRI was 0.708 (sensitivity 84% and specificity 92%) and for FIB-4 was 1.36 (sensitivity 76% and specificity 92%). Aspartate aminotransferase to Platelet Ratio Index and FIB-4 were less useful to predict cirrhosis. In contrast to recent reports from Europe and North America, both APRI and FIB-4 may identify significant fibrosis in HDV-III-infected patients from northwestern Brazil.
Conflict of interest statement
Disclosure: HW reports personal fees and non-financial support from Roche Diagnostics, personal fees and non-financial support from Abbott, personal fees and non-financial support from Eiger, non-financial support from MYR GmbH, outside the submitted work; and an honoraria for consulting and research support by companies developing diagnostic tools and antiviral therapies for viral hepatitis B and C.
Figures
References
-
- WHO , 2017. Global Hepatitis Report 2017. Available at: https://apps.who.int/iris/handle/10665/255016. Accessed August 15, 2018.
-
- Taylor JM, 2006. Structure and replication of hepatitis delta virus RNA. Curr Top Microbiol Immunol 307: 1–23. - PubMed
-
- Fonseca JC, Simonetti SR, Schatzmayr HG, Castejon MJ, Cesario AL, Simonetti JP, 1988. Prevalence of infection with hepatitis delta virus (HDV) among carriers of hepatitis B surface antigen in Amazonas State, Brazil. Trans R Soc Trop Med Hyg 82: 469–471. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

