Osteogenic and Chondrogenic Potential of the Supramolecular Aggregate T-LysYal®
- PMID: 32431670
- PMCID: PMC7214626
- DOI: 10.3389/fendo.2020.00285
Osteogenic and Chondrogenic Potential of the Supramolecular Aggregate T-LysYal®
Abstract
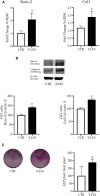
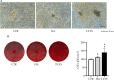
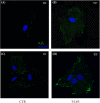
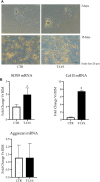
Hard tissue regeneration represents a challenge for the Regenerative Medicine and Mesenchymal stem cells (MSCs) could be a successful therapeutic strategy. T-LysYal® (T-Lys), a new derivative of Hyaluronic Acid (HA) possessing a superior stability, has already been proved efficient in repairing corneal epithelial cells damaged by dry conditions in vitro. We investigated the regenerative potential of T-Lys in the hard tissues bone and cartilage. We have previously demonstrated that cells isolated from the tooth germ, Dental Bud Stem Cells (DBSCs), differentiate into osteoblast-like cells, representing a promising source of MSCs for bone regeneration. Herewith, we show that T-Lys treatment stimulates the expression of typical osteoblastic markers, such as Runx-2, Collagen I (Col1) and Alkaline Phosphatase (ALP), determining a higher production of mineralized matrix nodules. In addition, we found that T-Lys treatment positively affects αVβ3 integrin expression, key integrin in the osteoblastic commitment, leading to the formation of focal adhesions (FAs). The efficacy of T-Lys was also tested on chondrogenic differentiation starting from human articular chondrocytes (HACs) resulting in an increase of differentiation markers and cell number.
Keywords: bone; cartilage; focal adhesions; mesenchymal stem cells; supramolecular aggregate; tissue regeneration.
Copyright © 2020 Di Benedetto, Posa, Marazzi, Kalemaj, Grassi, Lo Muzio, Comite, Cavalcanti-Adam, Grassi and Mori.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

