Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation
- PMID: 32431671
- PMCID: PMC7216689
- DOI: 10.3389/fmicb.2020.00517
Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation
Abstract
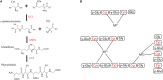
The persistence of heavy metals (HMs) in the environment causes adverse effects to all living organisms; HMs accumulate along the food chain affecting different levels of biological organizations, from cells to tissues. HMs enter cells through transporter proteins and can bind to enzymes and nucleic acids interfering with their functioning. Strategies used by microalgae to minimize HM toxicity include the biosynthesis of metal-binding peptides that chelate metal cations inhibiting their activity. Metal-binding peptides include genetically encoded metallothioneins (MTs) and enzymatically produced phytochelatins (PCs). A number of techniques, including genetic engineering, focus on increasing the biosynthesis of MTs and PCs in microalgae. The present review reports the current knowledge on microalgal MTs and PCs and describes the state of art of their use for HM bioremediation and other putative biotechnological applications, also emphasizing on techniques aimed at increasing the cellular concentrations of MTs and PCs. In spite of the broad metabolic and chemical diversity of microalgae that are currently receiving increasing attention by biotechnological research, knowledge on MTs and PCs from these organisms is still limited to date.
Keywords: cysteine; glutathione; heavy metals; metal-binding proteins; metallothioneins; microalgal biotechnologies; phycoremediation; phytochelatins.
Copyright © 2020 Balzano, Sardo, Blasio, Chahine, Dell’Anno, Sansone and Brunet.
Figures
References
-
- Adam V., Petrlova J., Potesil D., Zehnalek J., Sures B., Trnkova L., et al. (2005). Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 17 1649–1657. 10.1002/elan.200403264 - DOI
-
- Afonso C., Hathout Y., Fenselau C. (2004). Evidence for zinc ion sharing in metallothionein dimers provided by collision-induced dissociation. Int. J. Mass Spectrom. 231 207–211. 10.1016/j.ijms.2003.10.007 - DOI
-
- Ahner B. A., Kong S., Morel F. M. M. (1995). Phytochelatin production in marine algae. 1. An interspecific comparison. Limnol. Oceanogr. 40 649–657. 10.4319/lo.1995.40.4.0649 - DOI
-
- Ahner B. A., Morel F. M. M. (1995). Phytochelatin production in marine algae. 2. Induction by various metals. Limnol. Oceanogr. 40 658–665. 10.4319/lo.1995.40.4.0658 - DOI
Publication types
LinkOut - more resources
Full Text Sources

