Management of cytotoxic chemotherapy-induced hand-foot syndrome
- PMID: 32431787
- PMCID: PMC7232019
- DOI: 10.4081/oncol.2020.442
Management of cytotoxic chemotherapy-induced hand-foot syndrome
Abstract
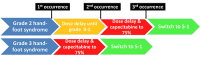
Improvements in systemic cancer treatments have resulted in more patients surviving for prolonged periods of time on treatment. This has made treatment-related toxicity and quality of life concerns increasingly relevant. Hand-foot syndrome (HFS) is a common skin reaction to systemic therapy that should be anticipated with chemotherapeutic treatments such as pegylated liposomal doxorubicin, docetaxel, and fluoropyrimidines. In this review we discuss current knowledge of the diagnosis, incidence, pathogenesis, and management of hand-foot syndrome (HFS). Although HFS is not life threatening, it can cause significant discomfort and impairment of function, especially in elderly patients, and may seriously impact quality of life. The incidence of HFS is dependent on the chemotherapeutic drug used, the treatment schedule, and the median duration of treatment. Effective measures for prevention and treatment of HFS include systemic and topical treatments, dose reductions, and switching to other drugs in the same class that are associated with lower rates of HFS. These approaches allow patients to continue cancer treatment while reducing negative impacts on quality of life. Awareness and early recognition are important to ensure timely treatment and avoidance of dose reductions or treatment discontinuation. We provide useful recommendations to guide the management of HFS in clinical practice.
Keywords: Hand-foot syndrome; docetaxel; doxorubicin; fluoropyrimidines; quality of life.
©Copyright: the Author(s).
Conflict of interest statement
Conflict of interests: Prof dr CJA Punt and prof dr M Koopman received research grants from the Dutch Colorectal Cancer Group and Servier outside the submitted work; dr JJM Kwakman received personal fees from Nordic Pharma for the conduct of this review, and research grants and lecture fees from Servier and Nordic Pharma outside the submitted work; dr YS Elshot received personal fees from Nordic Pharma for the conduct of this review. Nordic Pharma had no role in the design or writing of the review.
Figures
References
-
- Saif MW, Elfiky AA. Identifying and treating fluoropyrimidine- associated hand-and-foot syndrome in white and nonwhite patients. J Support Oncol 2007;7:337-43. - PubMed
-
- CTCAE v5 November 27, 2017. Common Terminology Criteria for Adverse Events. National Cancer Institute. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.....
-
- Van Doorn L, Veelenturf S, Binkhorst L, et al. Capecitabine and the Risk of Fingerprint Loss. JAMA Oncol 2017;3:122-3. - PubMed
-
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapyinduced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome: incidence, recognision and management. Am J Clin Dermatol 2000;1:225-34. - PubMed
-
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda). Eur J Oncol Nurs. 2004;8 Suppl 1:S31-40. - PubMed
LinkOut - more resources
Full Text Sources
Medical