A structural study of TatD from Staphylococcus aureus elucidates a putative DNA-binding mode of a Mg2+-dependent nuclease
- PMID: 32431834
- PMCID: PMC7201278
- DOI: 10.1107/S2052252520003917
A structural study of TatD from Staphylococcus aureus elucidates a putative DNA-binding mode of a Mg2+-dependent nuclease
Abstract
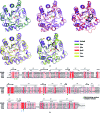
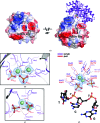
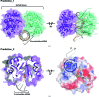
TatD has been thoroughly investigated as a DNA-repair enzyme and an apoptotic nuclease, and still-unknown TatD-related DNases are considered to play crucial cellular roles. However, studies of TatD from Gram-positive bacteria have been hindered by an absence of atomic detail and the resulting inability to determine function from structure. In this study, an X-ray crystal structure of SAV0491, which is the TatD enzyme from the Gram-positive bacterium Staphylococcus aureus (SaTatD), is reported at a high resolution of 1.85 Å with a detailed atomic description. Although SaTatD has the common TIM-barrel fold shared by most TatD-related homologs, and PDB entry 2gzx shares 100% sequence identity with SAV0491, the crystal structure of SaTatD revealed a unique binding mode of two phosphates interacting with two Ni2+ ions. Through a functional study, it was verified that SaTatD has Mg2+-dependent nuclease activity as a DNase and an RNase. In addition, structural comparison with TatD homologs and the identification of key residues contributing to the binding mode of Ni2+ ions and phosphates allowed mutational studies to be performed that revealed the catalytic mechanism of SaTatD. Among the key residues composing the active site, the acidic residues Glu92 and Glu202 had a critical impact on catalysis by SaTatD. Furthermore, based on the binding mode of the two phosphates and structural insights, a putative DNA-binding mode of SaTatD was proposed using in silico docking. Overall, these findings may serve as a good basis for understanding the relationship between the structure and function of TatD proteins from Gram-positive bacteria and may provide critical insights into the DNA-binding mode of SaTatD.
Keywords: DNA-binding protein; Staphylococcus aureus; TatD; X-ray crystallography; enzyme mechanisms; metal-dependent nuclease; protein structure; refinement; structure determination.
© Kyu-Yeon Lee et al. 2020.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

