Entropic bonding of the type 1 pilus from experiment and simulation
- PMID: 32431906
- PMCID: PMC7211842
- DOI: 10.1098/rsos.200183
Entropic bonding of the type 1 pilus from experiment and simulation
Abstract
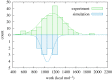
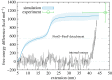
The type 1 pilus is a bacterial filament consisting of a long coiled proteic chain of subunits joined together by non-covalent bonding between complementing -strands. Its strength and structural stability are critical for its anchoring function in uropathogenic Escherichia coli bacteria. The pulling and unravelling of the FimG subunit of the pilus was recently studied by atomic force microscopy experiments and steered molecular dynamics simulations (Alonso-Caballero et al. 2018 Nat. Commun. 9, 2758. (doi:10.1038/s41467-018-05107-6)). In this work, we perform a quantitative comparison between experiment and simulation, showing a good agreement in the underlying work values for the unfolding. The simulation results are then used to estimate the free energy difference for the detachment of FimG from the complementing strand of the neighbouring subunit in the chain, FimF. Finally, we show that the large free energy difference for the unravelling and detachment of the subunits which leads to the high stability of the chain is entirely entropic in nature.
Keywords: atomic force microscopy; pilus; steered molecular dynamics.
© 2020 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Lavery R, Lebrun A, Allemand J-F, Bensimon D, Croquette V. 2002. Structure and mechanics of single biomolecules: experiment and simulation. J. Phys. Condens. Matter 14, R383–R414. ( 10.1088/0953-8984/14/14/202) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
