Urinary Morbidity in Men Treated With Stereotactic Body Radiation Therapy (SBRT) for Localized Prostate Cancer Following Transurethral Resection of the Prostate (TURP)
- PMID: 32432033
- PMCID: PMC7214538
- DOI: 10.3389/fonc.2020.00555
Urinary Morbidity in Men Treated With Stereotactic Body Radiation Therapy (SBRT) for Localized Prostate Cancer Following Transurethral Resection of the Prostate (TURP)
Abstract
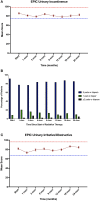
Background: Clinical data suggest that stereotactic body radiation therapy (SBRT) provides similar clinical outcomes as other radiation modalities for prostate cancer. However, data reporting on the safety of SBRT after TURP is limited. Herein, we report our experience using SBRT to deliver hypofractionated radiotherapy in patients with a history of TURP including physician-reported toxicities and patient-reported quality of life. Methods: Forty-seven patients treated with SBRT from 2007 to 2016 at Georgetown University Hospital for localized prostate carcinoma with a history of prior TURP were included in this retrospective analysis. Treatment was delivered using the CyberKnife® (Accuray Incorporated, Sunnyvale, CA) with doses of 35 Gy or 36.25 Gy in 5 fractions without prostatic urethral sparing. Toxicities were recorded and scored using the CTCAE v.4. Cystoscopy findings were retrospectively reviewed. Urinary quality of life data was assessed using the International Prostate Symptom Scoring (IPSS) and Expanded Prostate Cancer Index Composite 26 (EPIC-26). A Wilcoxon signed-rank sum test was used to determine if there was a statistically significant increase or decrease in IPSS or EPIC scores between timepoints. Minimally important differences were calculated by obtaining half the standard deviation at time of start of treatment. Results: Forty-seven patients at a median age of 72 years (range 63-84) received SBRT. The mean follow-up was 4.7 years (range 2-10 years). Late Grade 2 and grade 3 urinary toxicity occurred in 23 (48.9%) and 3 (6.4%) men, respectively. There were no Grade 4 or 5 toxicities. Approximately 51% of patients experienced hematuria following treatment. Mean time to hematuria was 10.5 months. Twenty-five cystoscopies were performed during follow-up and the most common finding was hyperemia, varices of the bladder neck/TURP defect, and/or necrotic tissue in the TURP defect. Baseline urinary QOL composite scores were low, but they did not clinically significantly decline in the first 2 years following treatment. Conclusions: In patients with prior TURP, prostate SBRT was well-tolerated. GU toxicity rates were comparable to similar patients treated with conventionally fractionated radiation therapy. Urinary quality of life was poor at baseline, but did not worsen clinically over time. Stricter dosimetric criteria could potentially improve the rate of high-grade late toxicity, but may increase the risk of peri-urethral recurrence.
Keywords: CyberKnife; EPIC-26; IPSS; SBRT; benign prostatic hyperplasia; common toxicity criteria (CTC); prostate cancer; quality of life.
Copyright © 2020 Pepin, Aghdam, Shah, Kataria, Tsou, Datta, Danner, Ayoob, Yung, Lei, Gurka, Collins, Krishnan, Suy, Hankins, Lynch and Collins.
Figures


Similar articles
-
Stereotactic Body Radiation Therapy (SBRT) for Prostate Cancer in Men With a High Baseline International Prostate Symptom Score (IPSS ≥ 15).Front Oncol. 2020 Jul 3;10:1060. doi: 10.3389/fonc.2020.01060. eCollection 2020. Front Oncol. 2020. PMID: 32719744 Free PMC article.
-
Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience.Radiat Oncol. 2013 Mar 13;8:58. doi: 10.1186/1748-717X-8-58. Radiat Oncol. 2013. PMID: 23497695 Free PMC article.
-
Hematuria following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer.Radiat Oncol. 2015 Feb 19;10:44. doi: 10.1186/s13014-015-0351-6. Radiat Oncol. 2015. PMID: 25890265 Free PMC article.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.Health Technol Assess. 2005 Feb;9(4):iii-iv, 1-30. doi: 10.3310/hta9040. Health Technol Assess. 2005. PMID: 15698525 Review.
-
Genitourinary toxicity in patients receiving TURP prior to hypofractionated radiotherapy for clinically localized prostate cancer: A scoping review.Urol Oncol. 2024 Jun;42(6):165-174. doi: 10.1016/j.urolonc.2024.02.011. Epub 2024 Mar 19. Urol Oncol. 2024. PMID: 38503591
Cited by
-
Extreme hypofractionated stereotactic radiotherapy for localized prostate Cancer: Efficacy and late urinary toxicity according to transurethral resection of the prostate history.Clin Transl Radiat Oncol. 2024 Apr 18;46:100779. doi: 10.1016/j.ctro.2024.100779. eCollection 2024 May. Clin Transl Radiat Oncol. 2024. PMID: 38681137 Free PMC article.
-
Stereotactic body radiation therapy for prostate cancer after surgical treatment of prostatic obstruction: Impact on urinary morbidity and mitigation strategies.Clin Transl Radiat Oncol. 2023 Dec 10;45:100709. doi: 10.1016/j.ctro.2023.100709. eCollection 2024 Mar. Clin Transl Radiat Oncol. 2023. PMID: 38179576 Free PMC article.
-
MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer.Cancers (Basel). 2021 Apr 9;13(8):1791. doi: 10.3390/cancers13081791. Cancers (Basel). 2021. PMID: 33918650 Free PMC article. Review.
-
Urethra-sparing prostate cancer radiotherapy: Current practices and future insights from an international survey.Clin Transl Radiat Oncol. 2024 Dec 30;51:100907. doi: 10.1016/j.ctro.2024.100907. eCollection 2025 Mar. Clin Transl Radiat Oncol. 2024. PMID: 39845565 Free PMC article.
-
Dosimetric Clues to Addressing Urinary Toxicity Following Stereotactic Prostate Radiation Therapy.Adv Radiat Oncol. 2025 Jul 3;10(9):101850. doi: 10.1016/j.adro.2025.101850. eCollection 2025 Sep. Adv Radiat Oncol. 2025. PMID: 40747327 Free PMC article.
References
-
- Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional vs hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, Phase 3 CHHiP trial. Lancet. (2016) 17:1047–60. 10.1016/S1470-2045(16)30102-4 - DOI - PMC - PubMed
-
- Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypogractionated vs conventionally fractionated radiotherapy for prostate cancer: 5-Year outcomes of the HYPO-RT-PC randomised, non-inferiority, Phase 3 Trial. Lancet. (2019) 394:383–95. 10.1016/S0140-6736(19)31131-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources

