Complement receptor 1 genetic polymorphism contributes to sporadic Alzheimer's disease susceptibility in Caucasians: a meta-analysis
- PMID: 32432316
- PMCID: PMC7268259
- DOI: 10.1042/BSR20200321
Complement receptor 1 genetic polymorphism contributes to sporadic Alzheimer's disease susceptibility in Caucasians: a meta-analysis
Abstract
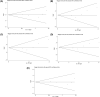
Complement receptor 1 (CR1) plays an important role in the development of sporadic Alzheimer's disease (SAD) in Caucasians. However, the influence of CR1 (rs6656401A/G and rs3818361T/C) genetic polymorphisms on the risk of SAD remains controversial. A meta-analysis of 18 case-control studies was performed to derive a more precise association of CR1 (rs6656401A/G or rs3818361T/C) genetic polymorphism with the risk of SAD in Caucasians. A statistical difference was found in the dominant model (odds ratio (OR): 1.23, 95% confidence interval (CI): 1.16-1.30, P=0.00), recessive model (OR: 1.28, 95% CI: 1.05-1.56, P=0.02), homozygote comparison (OR: 1.36, 95% CI: 1.12-1.66, P=0.002) or heterozygote comparison (AG versus GG) (OR: 1.21, 95% CI: 1.15-1.29, P=0.00) of CR1 rs6656401A/G. For CR1 rs3818361T/C, a statistical difference was observed in the dominant model (OR: 1.21, 95% CI: 1.13-1.31, P=0.00), recessive model (OR: 1.28, 95% CI: 1.07-1.53, P=0.006), homozygote comparison (OR: 1.35, 95% CI: 1.13-1.62, P=0.001) or heterozygote comparison (TC versus CC) (OR: 1.20, 95% CI: 1.11-1.29, P=0.00). In summary, despite some limitations, the present meta-analysis indicated that rs6656401A/G or rs3818361T/C polymorphism was related to SAD risk. Moreover, a carrier of rs6656401A/G or T carrier of rs3818361T/C in CR1 genetic polymorphism might be an increased factor for SAD in Caucasians.
Keywords: Alzheimer's disease; complement receptor 1; gene; meta-analysis; polymorphism.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures







Similar articles
-
GAB2 polymorphism rs2373115 confers susceptibility to sporadic Alzheimer's disease.Neurosci Lett. 2013 Nov 27;556:216-20. doi: 10.1016/j.neulet.2013.10.036. Epub 2013 Oct 22. Neurosci Lett. 2013. PMID: 24161894
-
Meta-analysis of the association between CR1 polymorphisms and risk of late-onset Alzheimer's disease.Neurosci Lett. 2014 Aug 22;578:165-70. doi: 10.1016/j.neulet.2014.06.055. Epub 2014 Jul 1. Neurosci Lett. 2014. PMID: 24996192
-
Association of the CR1 polymorphism with late-onset Alzheimer's disease in Chinese Han populations: a meta-analysis.Neurosci Lett. 2012 Oct 3;527(1):46-9. doi: 10.1016/j.neulet.2012.08.032. Epub 2012 Aug 27. Neurosci Lett. 2012. PMID: 22960360
-
CR1 in Alzheimer's disease.Mol Neurobiol. 2015 Apr;51(2):753-65. doi: 10.1007/s12035-014-8723-8. Epub 2014 May 4. Mol Neurobiol. 2015. PMID: 24794147 Review.
-
Interleukin-23R rs7517847 T/G Polymorphism Contributes to the Risk of Crohn's Disease in Caucasians: A Meta-Analysis.J Immunol Res. 2015;2015:279849. doi: 10.1155/2015/279849. Epub 2015 May 18. J Immunol Res. 2015. PMID: 26090488 Free PMC article. Review.
Cited by
-
Immune cells in Alzheimer's disease: insights into pathogenesis and potential therapeutic targets.Med Rev (2021). 2024 Dec 23;5(3):179-202. doi: 10.1515/mr-2024-0064. eCollection 2025 Jun. Med Rev (2021). 2024. PMID: 40600183 Free PMC article. Review.
-
A Review of the Knops Blood Group System.Clin Appl Thromb Hemost. 2024 Jan-Dec;30:10760296241309638. doi: 10.1177/10760296241309638. Clin Appl Thromb Hemost. 2024. PMID: 39706812 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

