Pharmacogenetic assessment of tafenoquine efficacy in patients with Plasmodium vivax malaria
- PMID: 32433338
- PMCID: PMC7409770
- DOI: 10.1097/FPC.0000000000000407
Pharmacogenetic assessment of tafenoquine efficacy in patients with Plasmodium vivax malaria
Abstract
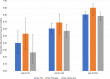
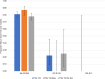
Plasmodium vivax has the largest geographic range of human malaria species and is challenging to manage and eradicate due to its ability to establish a dormant liver stage, the hypnozoite, which can reactivate leading to relapse. Until recently, the only treatment approved to kill hypnozoites was the 8-aminoquinoline, primaquine, requiring daily treatment for 14 days. Tafenoquine, an 8-aminoquinoline single-dose treatment with activity against P. vivax hypnozoites, has recently been approved by the US Food and Drug Administration and Australian Therapeutic Goods Administration for the radical cure of P. vivax malaria in patients 16 years and older. We conducted an exploratory pharmacogenetic analysis (GSK Study 208099) to assess the role of host genome-wide variation on tafenoquine efficacy in patients with P. vivax malaria using data from three GSK clinical trials, GATHER and DETECTIVE Part 1 and Part 2. Recurrence-free efficacy at 6 and 4 months and time to recurrence up to 6 months postdosing were analyzed in 438 P. vivax malaria patients treated with tafenoquine. Among the approximately 10.6 million host genetic variants analyzed, two signals reached genome-wide significance (P value ≤ 5 × 10). rs62103056, and variants in a chromosome 12 intergenic region, were associated with recurrence-free efficacy at 6 and 4 months, respectively. Neither of the signals has an obvious biological rationale and would need replication in an independent population. This is the first genome-wide association study to evaluate genetic influence on response to tafenoquine in P. vivax malaria.
Conflict of interest statement
The views expressed here are solely those of the authors and do not reflect the views, policies or positions of the U.S. Government or Department of Defense. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human participants as prescribed in AR 70–25.
D.P. declares grants and nonfinancial support from GSK and MMV. A.L-C. declares grants and personal fees from GSK. D.Y. declares grants from GSK. J.B. and G.K. are GSK employees and hold shares/options in GSK. J.G. is a ViiV employee and holds shares/options in GSK. S.D. is an MMV employee. P.S. is an employee at Parexel whose work was funded by a contract with GSK. There are no conflicts of interest for the remaining authors.
Figures
References
-
- World Health Organization. Guidelines for the Treatment of Malaria. 20153rd ed, Geneva: World Health Organization - PubMed
-
- Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014; 383:1049–1058 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

