A guide to cancer immunotherapy: from T cell basic science to clinical practice
- PMID: 32433532
- PMCID: PMC7238960
- DOI: 10.1038/s41577-020-0306-5
A guide to cancer immunotherapy: from T cell basic science to clinical practice
Abstract
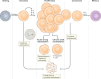
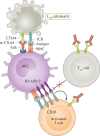
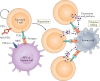
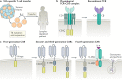
The T lymphocyte, especially its capacity for antigen-directed cytotoxicity, has become a central focus for engaging the immune system in the fight against cancer. Basic science discoveries elucidating the molecular and cellular biology of the T cell have led to new strategies in this fight, including checkpoint blockade, adoptive cellular therapy and cancer vaccinology. This area of immunological research has been highly active for the past 50 years and is now enjoying unprecedented bench-to-bedside clinical success. Here, we provide a comprehensive historical and biological perspective regarding the advent and clinical implementation of cancer immunotherapeutics, with an emphasis on the fundamental importance of T lymphocyte regulation. We highlight clinical trials that demonstrate therapeutic efficacy and toxicities associated with each class of drug. Finally, we summarize emerging therapies and emphasize the yet to be elucidated questions and future promise within the field of cancer immunotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures

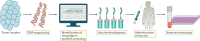
References
-
- Oiseth SJ, Aziz MSA-E. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017;3:250–261.
-
- Decker WK, Safdar A. Bioimmunoadjuvants for the treatment of neoplastic and infectious disease: Coley’s legacy revisited. Cytokine Growth Factor. Rev. 2009;20:271–281. - PubMed
-
- Gross L. Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer Res. 1943;3:326.
-
- Smith JL, Jr., Stehlin JS., Jr. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965;18:1399–1415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

