Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses
- PMID: 32433540
- PMCID: PMC7264306
- DOI: 10.1038/s41423-020-0465-0
Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses
Abstract
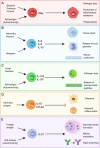
Dendritic cells are powerful antigen-presenting cells that are essential for the priming of T cell responses. In addition to providing T-cell-receptor ligands and co-stimulatory molecules for naive T cell activation and expansion, dendritic cells are thought to also provide signals for the differentiation of CD4+ T cells into effector T cell populations. The mechanisms by which dendritic cells are able to adapt and respond to the great variety of infectious stimuli they are confronted with, and prime an appropriate CD4+ T cell response, are only partly understood. It is known that in the steady-state dendritic cells are highly heterogenous both in phenotype and transcriptional profile, and that this variability is dependent on developmental lineage, maturation stage, and the tissue environment in which dendritic cells are located. Exposure to infectious agents interfaces with this pre-existing heterogeneity by providing ligands for pattern-recognition and toll-like receptors that are variably expressed on different dendritic cell subsets, and elicit production of cytokines and chemokines to support innate cell activation and drive T cell differentiation. Here we review current information on dendritic cell biology, their heterogeneity, and the properties of different dendritic cell subsets. We then consider the signals required for the development of different types of Th immune responses, and the cellular and molecular evidence implicating different subsets of dendritic cells in providing such signals. We outline how dendritic cell subsets tailor their response according to the infectious agent, and how such transcriptional plasticity enables them to drive different types of immune responses.
Keywords: CD4+ T cells; Th effector responses; dendritic cells.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Patente TA, Pelgrom LR, Everts B. Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization. Curr. Opin. Immunol. 2019;58:16–23. - PubMed
-
- Blecher-Gonen R, et al. Single-cell analysis of diverse pathogen responses defines a molecular roadmap for generating antigen-specific immunity. Cell Syst. 2019;8:109–121 e106. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

