Sexual modulation in a polyploid grass: a reproductive contest between environmentally inducible sexual and genetically dominant apomictic pathways
- PMID: 32433575
- PMCID: PMC7239852
- DOI: 10.1038/s41598-020-64982-6
Sexual modulation in a polyploid grass: a reproductive contest between environmentally inducible sexual and genetically dominant apomictic pathways
Abstract
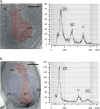
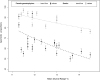
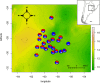
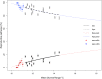
In systems alternating between sexual and asexual reproduction, sex increases under unfavorable environmental conditions. In plants producing sexual and asexual (apomictic) seeds, studies on the influence of environmental factors on sex are equivocal. We used Paspalum intermedium to study environmental effects on the expression of sexual and apomictic developments, and on resulting reproductive fitness variables. Flow cytometric and embryological analyses were performed to characterize ploidy and reproductive modes, and effects of local climatic conditions on sexual and apomictic ovule and seed frequencies were determined. Seed set and germination data were collected and used to estimate reproductive fitness. Frequencies of sexual and apomictic ovules and seeds were highly variable within and among populations. Apomictic development exhibited higher competitive ability but lower overall fitness. Frequencies of sexual reproduction in facultative apomictic plants increased at lower temperatures and wider mean diurnal temperature ranges. We identified a two-fold higher fitness advantage of sexuality and a Tug of War between factors intrinsic to apomixis and environmental stressors promoting sexuality which influence the distribution of sex in apomictic populations. This points toward a crucial role of local ecological conditions in promoting a reshuffling of genetic variability that may be shaping the adaptative landscape in apomictic P. intermedium plants.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Competition between meiotic and apomictic pathways during ovule and seed development results in clonality.New Phytol. 2013 Jan;197(1):336-347. doi: 10.1111/j.1469-8137.2012.04381.x. Epub 2012 Nov 5. New Phytol. 2013. PMID: 23127139
-
Small RNA-seq reveals novel regulatory components for apomixis in Paspalum notatum.BMC Genomics. 2019 Jun 13;20(1):487. doi: 10.1186/s12864-019-5881-0. BMC Genomics. 2019. PMID: 31195966 Free PMC article.
-
PnTgs1-like expression during reproductive development supports a role for RNA methyltransferases in the aposporous pathway.BMC Plant Biol. 2014 Nov 18;14:297. doi: 10.1186/s12870-014-0297-0. BMC Plant Biol. 2014. PMID: 25404464 Free PMC article.
-
Harnessing apomictic reproduction in grasses: what we have learned from Paspalum.Ann Bot. 2013 Sep;112(5):767-87. doi: 10.1093/aob/mct152. Epub 2013 Jul 17. Ann Bot. 2013. PMID: 23864004 Free PMC article. Review.
-
The genetic control of apomixis: asexual seed formation.Genetics. 2014 Jun;197(2):441-50. doi: 10.1534/genetics.114.163105. Genetics. 2014. PMID: 24939990 Free PMC article. Review.
Cited by
-
New Frontiers in Potato Breeding: Tinkering with Reproductive Genes and Apomixis.Biomolecules. 2024 May 23;14(6):614. doi: 10.3390/biom14060614. Biomolecules. 2024. PMID: 38927018 Free PMC article. Review.
-
Alien genome mobilization and fixation utilizing an apomixis mediated genome addition (AMGA) strategy in Pennisetum to improve domestication traits of P. squamulatum.Theor Appl Genet. 2022 Jul;135(7):2555-2575. doi: 10.1007/s00122-022-04138-4. Epub 2022 Jun 20. Theor Appl Genet. 2022. PMID: 35726065
-
Apomixis Technology: Separating the Wheat from the Chaff.Genes (Basel). 2020 Apr 10;11(4):411. doi: 10.3390/genes11040411. Genes (Basel). 2020. PMID: 32290084 Free PMC article.
-
Can We Use Gene-Editing to Induce Apomixis in Sexual Plants?Genes (Basel). 2020 Jul 12;11(7):781. doi: 10.3390/genes11070781. Genes (Basel). 2020. PMID: 32664641 Free PMC article.
-
Variation of Residual Sexuality Rates along Reproductive Development in Apomictic Tetraploids of Paspalum.Plants (Basel). 2022 Jun 21;11(13):1639. doi: 10.3390/plants11131639. Plants (Basel). 2022. PMID: 35807591 Free PMC article.
References
-
- Suomalainen, E., Saura, A. & Lokki, J. Cytology and evolution in parthenogenesis. (CRC Press (1987).
-
- Asker, S. & Jerling, L. Apomixis in plants. (CRC Press (1992).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

