Clinical outcomes of older patients with AML receiving hypomethylating agents: a large population-based study in the United States
- PMID: 32433746
- PMCID: PMC7252544
- DOI: 10.1182/bloodadvances.2020001779
Clinical outcomes of older patients with AML receiving hypomethylating agents: a large population-based study in the United States
Abstract
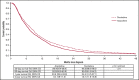
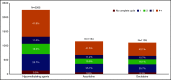
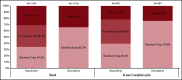
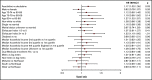
The hypomethylating agents (HMAs) azacitidine and decitabine have been the de facto standard of care for patients with acute myeloid leukemia (AML) who are unfit for intensive therapy. Using the Surveillance, Epidemiology, and End Results-Medicare linked database, we identified 2263 older adults (age ≥66 years) diagnosed with AML during 2005-2015 who received a first-line HMA; 1154 (51%) received azacitidine, and 1109 (49%) received decitabine. Median survival from diagnosis was 7.1 and 8.2 months (P < .01) for azacitidine- and decitabine-treated patients, respectively. Mortality risk was higher with azacitidine vs decitabine (hazard ratio [HR], 1.11; 95% confidence interval [CI], 1.01-1.21; P = .02). The findings were similar when evaluating only patients completing ≥4 cycles (42% of patients treated with either azacitidine or decitabine). These findings lost significance when evaluating those completing a standard 7-day schedule of azacitidine (34%) vs 5-day schedule for decitabine (66%) (HR, 0.95; 95% CI, 0.83-1.08; P = .43). Red blood cell (RBC) transfusion independence (TI) was achieved in one-third of patients with no difference between the 2 HMAs. In conclusion, the majority of older AML patients did not receive the minimum of 4 cycles of HMA often needed to elicit clinical benefit. We observed no clinically meaningful differences between azacitidine- and decitabine-treated patients in their achievement of RBC TI or survival.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.M.Z. received research funding (institutional) from Celgene, Acceleron, AbbVie, Novartis, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics; had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Ariad, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, Ionis, Epizyme, and Takeda; and received travel support for meetings from Pfizer, Novartis, and Trovagene. R.W. received research funding from Celgene Corp. S.D.G. received research support from Celgene and Boehringer-Ingelheim and consulted for AbbVie. A.J.D. received research funding from Celgene Corp. X.M. received research funding (institutional) from Celgene Corporation for the work included in this article. The remaining authors declare no competing financial interests.
Figures




References
-
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70-87. - PubMed
-
- Shallis RM, Boddu PC, Bewersdorf JP, Zeidan AM. The golden age for patients in their golden years: the progressive upheaval of age and the treatment of newly-diagnosed acute myeloid leukemia. Blood Rev. 2020;40:100639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

