Autofluorescent Granules of the Human Retinal Pigment Epithelium: Phenotypes, Intracellular Distribution, and Age-Related Topography
- PMID: 32433758
- PMCID: PMC7405767
- DOI: 10.1167/iovs.61.5.35
Autofluorescent Granules of the Human Retinal Pigment Epithelium: Phenotypes, Intracellular Distribution, and Age-Related Topography
Abstract
Purpose: The human retinal pigment epithelium (RPE) accumulates granules significant for autofluorescence imaging. Knowledge of intracellular accumulation and distribution is limited. Using high-resolution microscopy techniques, we determined the total number of granules per cell, intracellular distribution, and changes related to retinal topography and age.
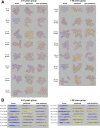
Methods: RPE cells from the fovea, perifovea, and near-periphery of 15 human RPE flat mounts were imaged using structured illumination microscopy (SIM) and confocal fluorescence microscopy in young (≤51 years, n = 8) and older (>80 years, n = 7) donors. Using custom FIJI plugins, granules were marked with computer assistance, classified based on morphological and autofluorescence properties, and analyzed with regard to intracellular distribution, total number per cell, and granule density.
Results: A total of 193,096 granules in 450 RPE cell bodies were analyzed. Based on autofluorescence properties, size, and composition, the RPE granules exhibited nine different phenotypes (lipofuscin, two; melanolipofuscin, five; melanosomes, two), distinguishable by SIM. Overall, lipofuscin (low at the fovea but increases with eccentricity and age) and melanolipofuscin (equally distributed at all three locations with no age-related changes) were the major granule types. Melanosomes were under-represented due to suboptimal visualization of apical processes in flat mounts.
Conclusions: Low lipofuscin and high melanolipofuscin content within foveal RPE cell bodies and abundant lipofuscin at the perifovea suggest a different genesis, plausibly related to the population of overlying photoreceptors (fovea, cones only; perifovea, highest rod density). This systematic analysis provides further insight into RPE cell and granule physiology and links granule load to cell autofluorescence, providing a subcellular basis for the interpretation of clinical fundus autofluorescence.
Conflict of interest statement
Disclosure:
Figures






References
-
- Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001; 2: 867–872. - PubMed
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85: 845–881. - PubMed
-
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978; 17: 583–600. - PubMed
-
- Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995; 105: 3–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

