Microtubule Nucleation Properties of Single Human γTuRCs Explained by Their Cryo-EM Structure
- PMID: 32433913
- PMCID: PMC7280788
- DOI: 10.1016/j.devcel.2020.04.019
Microtubule Nucleation Properties of Single Human γTuRCs Explained by Their Cryo-EM Structure
Abstract
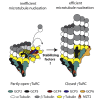
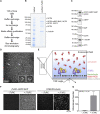
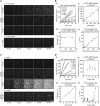
The γ-tubulin ring complex (γTuRC) is the major microtubule nucleator in cells. The mechanism of its regulation is not understood. We purified human γTuRC and measured its nucleation properties in a total internal reflection fluorescence (TIRF) microscopy-based real-time nucleation assay. We find that γTuRC stably caps the minus ends of microtubules that it nucleates stochastically. Nucleation is inefficient compared with microtubule elongation. The 4 Å resolution cryoelectron microscopy (cryo-EM) structure of γTuRC, combined with crosslinking mass spectrometry analysis, reveals an asymmetric conformation with only part of the complex in a "closed" conformation matching the microtubule geometry. Actin in the core of the complex, and MZT2 at the outer perimeter of the closed part of γTuRC appear to stabilize the closed conformation. The opposite side of γTuRC is in an "open," nucleation-incompetent conformation, leading to a structural asymmetry explaining the low nucleation efficiency of purified human γTuRC. Our data suggest possible regulatory mechanisms for microtubule nucleation by γTuRC closure.
Keywords: CLMS; MZT2; TIRF microscopy; TPX2; actin; chTOG; cryo-electron microscopy; microtubule nucleation; γ-tubulin ring complex; γTuRC structure.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Comment in
-
Nucleating a Microtubule: Put a γ-Tubulin Ring on It.Dev Cell. 2020 Jun 8;53(5):495-497. doi: 10.1016/j.devcel.2020.05.018. Dev Cell. 2020. PMID: 32516593
References
-
- Abella J.V., Galloni C., Pernier J., Barry D.J., Kjær S., Carlier M.F., Way M. Isoform diversity in the Arp2/3 complex determines actin filament dynamics. Nat. Cell Biol. 2016;18:76–86. - PubMed
-
- Akhmanova A., Steinmetz M.O. Control of microtubule organization and dynamics: two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015;16:711–726. - PubMed
-
- Aldaz H., Rice L.M., Stearns T., Agard D.A. Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature. 2005;435:523–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

