β-Glucan Induces Protective Trained Immunity against Mycobacterium tuberculosis Infection: A Key Role for IL-1
- PMID: 32433977
- PMCID: PMC7242907
- DOI: 10.1016/j.celrep.2020.107634
β-Glucan Induces Protective Trained Immunity against Mycobacterium tuberculosis Infection: A Key Role for IL-1
Abstract
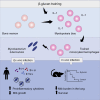
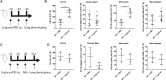
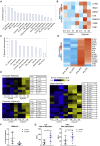
β-glucan is a potent inducer of epigenetic and functional reprogramming of innate immune cells, a process called "trained immunity," resulting in an enhanced host response against secondary infections. We investigate whether β-glucan exposure confers protection against pulmonary Mycobacterium tuberculosis (Mtb) infection. β-glucan induces trained immunity via histone modifications at gene promoters in human monocytes, which is accompanied by the enhanced production of proinflammatory cytokines upon secondary Mtb challenge and inhibition of Mtb growth. Mice treated with β-glucan are significantly protected against pulmonary Mtb infection, which is associated with the expansion of hematopoietic stem and progenitor cells in the bone marrow and increased myelopoiesis. The protective signature of β-glucan is mediated via IL-1 signaling, as β-glucan shows no protection in mice lacking a functional IL-1 receptor (IL1R-/-). The administration of β-glucan may be used as a novel strategy in the treatment of mycobacterial infections and possibly as an adjuvant to improve anti-tuberculosis vaccines.
Keywords: IL-1; Mycobacterium tuberculosis; epigenetics; innate immune memory; monocytes; trained immunity; tuberculosis; β-glucan.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





References
-
- Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89–100.e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

