Altered IL-32 Signaling in Abdominal Aortic Aneurysm
- PMID: 32434199
- PMCID: PMC7446302
- DOI: 10.1159/000507667
Altered IL-32 Signaling in Abdominal Aortic Aneurysm
Abstract
Introduction and objective: Interleukin (IL)-32 is a pro-inflammatory cytokine not previously studied in relation to abdominal aortic aneurysm (AAA). The aim of this study was to elucidate the expression and localization of IL-32 in AAA.
Methods: Expression and localization of IL-32 in human aortic tissue was studied with immunohistochemical analysis and Western blot (AAA: n = 5; controls: n = 4). ELISA was used to measure IL-32 in human plasma samples (AAA: n = 140; controls: n = 37) and in media from cultured peripheral blood mononuclear cells (PBMCs) from 3 healthy donors. IL-32 mRNA in PBMCs, endothelial cells, aortic smooth muscle cells (SMCs), and aortic tissue samples of AAA (n = 16) and control aortas (n = 9) was measured with qPCR.
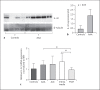
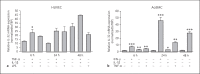
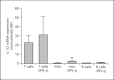
Results: IL-32 was predominantly expressed in SMCs and T-cell-rich areas. Highest mRNA expression was observed in the intima/media layer of the AAA. A weaker protein expression was detected in non-aneurysmal aortas. Expression of IL-32 was confirmed in isolated T cells, macrophages, endothelial cells, and SMCs, where expression was also inducible by cytokines such as interferon-γ. There was no difference in IL-32 expression in plasma between patients and controls.
Conclusion: IL-32 signaling is altered locally in AAA and could potentially play an important role in aneurysm development. Further studies using animal models would be helpful to study its potential role in AAA disease.
Keywords: Abdominal aortic aneurysm; Cytokines; Inflammation; Leukocytes; T cells.
© 2020 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41((Suppl 1)):S1–58. - PubMed
-
- Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67((1)):2–77.e2. - PubMed
-
- Budtz-Lilly J, Björck M, Venermo M, Debus S, Behrendt CA, Altreuther M, et al. Editor's choice: the impact of centralisation and endovascular aneurysm repair on treatment of ruptured abdominal aortic aneurysms based on international registries. Eur J Vasc Endovasc Surg. 2018;56((2)):181–8. - PubMed
-
- Björck M, Wanhainen A. Pathophysiology of AAA: heredity vs. environment. Prog Cardiovasc Dis. 2013;56((1)):2–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

