Resurrecting the Mysteries of Big Tau
- PMID: 32434664
- PMCID: PMC7999525
- DOI: 10.1016/j.tins.2020.04.007
Resurrecting the Mysteries of Big Tau
Abstract
Tau, a microtubule-associated protein that modifies the dynamic properties and organization of microtubules in neurons and affects axonal transport, shows remarkable heterogeneity, with multiple isoforms (45-65 kDa) generated by alternative splicing. A high-molecular-weight (HMW) isoform (110 kDa) that contains an additional large exon termed 4a was discovered more than 25 years ago. This isoform, called Big tau, is expressed mainly in the adult peripheral nervous system (PNS), but also in adult neurons of the central nervous system (CNS) that extend processes into the periphery. Surprisingly little has been learned about Big tau since its initial characterization, leaving a significant gap in knowledge about how the dramatic switch to Big tau affects the properties of neurons in the context of development, disease, or injury. Here we review what was learned about the structure and distribution of Big tau in those earlier studies, and add contemporary insights to resurrect interest in the mysteries of Big tau and thereby set a path for future studies.
Keywords: Alzheimer's disease; Big tau; axon; microtubule; neuron; peripheral nervous system; tau; tauopathy.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures




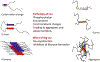
References
-
- Montejo de Garcini E et al. (1994) Overexpression of tau protein in COS-1 cells results in the stabilization of centrosome-independent microtubules and extension of cytoplasmic processes. Mol. Cell. Biochem 130, 187–196 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

