Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the Rapid Detection of SARS-CoV-2
- PMID: 32434706
- PMCID: PMC7217789
- DOI: 10.1016/j.jcv.2020.104428
Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the Rapid Detection of SARS-CoV-2
Abstract
Background: The SARS-CoV-2 pandemic has created an urgent and unprecedented need for rapid large-scale diagnostic testing to inform timely patient management. However, robust data are lacking on the relative performance of available rapid molecular tests across a full range of viral concentrations.
Objective: This study aimed to compare two recently-authorized rapid tests, Cepheid Xpert Xpress SARS-CoV-2 and Abbott ID Now SARS-CoV-2, to the Roche cobas SARS-CoV-2 assay for samples with low, medium, and high viral concentrations.
Study design: A total of 113 nasopharyngeal swabs from remnant patient samples were tested, including 88 positives spanning the full range of observed Ct values on the cobas assay.
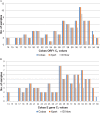
Results: Compared to cobas, the overall positive agreement was 73.9% with ID Now and 98.9% with Xpert. Negative agreement was 100% and 92.0% for ID Now and Xpert, respectively. Both ID Now and Xpert showed 100% positive agreement for medium and high viral concentrations (Ct value <30). However, for Ct values >30, positive agreement was 34.3% for ID Now and 97.1% for Xpert.
Conclusions: While Xpert showed high agreement with cobas across a wide range of viral concentrations, this study highlights an important limitation of ID Now for specimens collected in viral or universal transport media with low viral concentrations. Further studies are needed to evaluate the performance of ID Now for direct swabs.
Keywords: COVID-19; Molecular Diagnostics; PCR; Point of Care; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
References
-
- Sheridan C. Coronavirus and the race to distribute reliable diagnostics. Nature Biotechnology. 2020;38:382–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

