EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update
- PMID: 32434812
- PMCID: PMC7286048
- DOI: 10.1136/annrheumdis-2020-217159
EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update
Abstract
Objective: To update the European League Against Rheumatism (EULAR) recommendations for the pharmacological treatment of psoriatic arthritis (PsA).
Methods: According to the EULAR standardised operating procedures, a systematic literature review was followed by a consensus meeting to develop this update involving 28 international taskforce members in May 2019. Levels of evidence and strengths of recommendations were determined.
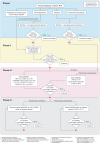
Results: The updated recommendations comprise 6 overarching principles and 12 recommendations. The overarching principles address the nature of PsA and diversity of both musculoskeletal and non-musculoskeletal manifestations; the need for collaborative management and shared decision-making is highlighted. The recommendations provide a treatment strategy for pharmacological therapies. Non-steroidal anti-inflammatory drugs and local glucocorticoid injections are proposed as initial therapy; for patients with arthritis and poor prognostic factors, such as polyarthritis or monoarthritis/oligoarthritis accompanied by factors such as dactylitis or joint damage, rapid initiation of conventional synthetic disease-modifying antirheumatic drugs is recommended. If the treatment target is not achieved with this strategy, a biological disease-modifying antirheumatic drugs (bDMARDs) targeting tumour necrosis factor (TNF), interleukin (IL)-17A or IL-12/23 should be initiated, taking into account skin involvement if relevant. If axial disease predominates, a TNF inhibitor or IL-17A inhibitor should be started as first-line disease-modifying antirheumatic drug. Use of Janus kinase inhibitors is addressed primarily after bDMARD failure. Phosphodiesterase-4 inhibition is proposed for patients in whom these other drugs are inappropriate, generally in the context of mild disease. Drug switches and tapering in sustained remission are addressed.
Conclusion: These recommendations provide stakeholders with an updated consensus on the pharmacological management of PsA, based on a combination of evidence and expert opinion.
Keywords: DMARDs (biologic); psoriatic arthritis; treatment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LG: AbbVie, Biogen, Celgene, Janssen, Lilly, Mylan, Novartis, Pfizer, Sandoz, Sanofi-Aventis, UCB. XB: AbbVie, Amgen, BMS, Celgene, Chugai, Hexal, Janssen, Lilly, MSD, Mylan, Novartis, Pfizer, Sandoz, UCB. AK: Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Merck Sharp and Dohme, Novartis, Pfizer. MdW: Through Stichting Tools from AbbVie, BMS, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Pfizer, Roche. IM: AbbVie, BMS, Lilly, Novartis, Celgene, Gilead, Janssen, Boehringer, UCB, Pfizer. MD: AbbVie, BMS, Janssen, Lilly, Novartis, Merck, Pfizer, UCB. JP: BMS, Pfizer. DGM: AbbVie, BMS, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, UCB. DA: AbbVie, Amgen, Gilead, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz, Sanofi/Genzyme, Sobi. AB: AbbVie, Amgen, AstraZeneca, Angelini, AlfaSigma, BMS, Berlin-Chemie, Egis, Ewopharma, GSK, Lilly, Mylan, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, Teva, UCB, Zentiva. PVB: AbbVie, Celgene, Lilly, MSD, Novartis, Pfizer, Richter. HB: Pfizer. W-HB: AbbVie, Almirall, BMS, Celgene, Leo, Lilly, Novartis, Pfizer, UCB. GRB: AbbVie, Celgene, Lilly, MSD, Novartis, Pfizer. JDC: Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, UCB. NSD: AbbVie, Boehringer Ingelheim, Gedeon Richter, Lilly, Novartis, Pfizer, Roche. TWK: Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, UCB. TKK: AbbVie, Amgen, Biogen, BMS, Celltrion, Egis, Eli Lilly, Ewopharma, Hikma, Hospira/Pfizer, MSD, Mylan, Orion Pharma, Roche, Sandoz, Sanofi, UCB. RBML: AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, MSD, Novartis, Pfizer, UCB. RJUL is Director of Rheumatology Consultancy; AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, UCB. HM-O: AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Takeda, UCB. DP: AbbVie, BMS, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, UCB. SARM: Janssen, MSD, Novartis. GS: AbbVie, BMS, Celgene, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, UCB. DJV: AbbVie, Biogen, Boehringer Ingelheim, HealthBeacon, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, UCB. FEVdB: AbbVie, Celgene, Eli Lilly, Galapagos/Gilead, Janssen, Merck, Novartis, Pfizer, UCB. DvdH: AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma; Director of Imaging Rheumatology. JSS: grants to institution from AbbVie, AstraZeneca, Janssen, Lilly, Merck Sharp & Dohme, Pfizer and Roche; speaker for AbbVie, Amgen, AstraZeneca, Astro, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Gilead, ILTOO Pharma, Janssen, Lilly, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche, Samsung, Sanofi and UCB.
Figures
Comment in
-
Response to: 'Comment on: 'EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update' by Gossec et al' by Wei et al.Ann Rheum Dis. 2022 Aug;81(8):e139. doi: 10.1136/annrheumdis-2020-218456. Epub 2020 Sep 1. Ann Rheum Dis. 2022. PMID: 32873555 No abstract available.
-
Comment on: 'EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update' by Gossec et al.Ann Rheum Dis. 2022 Aug;81(8):e138. doi: 10.1136/annrheumdis-2020-218385. Epub 2020 Sep 1. Ann Rheum Dis. 2022. PMID: 32873556 No abstract available.
References
-
- Fernández‐Carballido C, Martín‐Martínez MA, García‐Gómez C, et al. Impact of comorbidity on physical function in patients with ankylosing spondylitis and psoriatic arthritis attending rheumatology clinics. Results from the CAR diovascular in rheu MA tology (CARMA) study. Arthritis Care Res. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

