RocA Regulates Phosphatase Activity of Virulence Sensor CovS of Group A Streptococcus in Growth Phase- and pH-Dependent Manners
- PMID: 32434842
- PMCID: PMC7380576
- DOI: 10.1128/mSphere.00361-20
RocA Regulates Phosphatase Activity of Virulence Sensor CovS of Group A Streptococcus in Growth Phase- and pH-Dependent Manners
Abstract
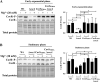
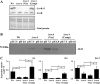
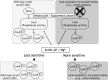
The control of the virulence response regulator and sensor (CovR-CovS) two-component regulatory system in group A Streptococcus (GAS) strains regulates more than 15% of gene expression and has critical roles in invasive GAS infection. The membrane-embedded CovS has kinase and phosphatase activities, and both are required for modulating the phosphorylation level of CovR. Regulator of Cov (RocA) is a positive regulator of covR and also been shown to be a pseudokinase that interacts with CovS to enhance the phosphorylation level of CovR; however, how RocA modulates the activity of CovS has not been determined conclusively. Although the phosphorylation level of CovR was decreased in the rocA mutant in the exponential phase, the present study shows that phosphorylated CovR in the rocA mutant increased to levels similar to those in the wild-type strain in the stationary phase of growth. In addition, acidic stress, which is generally present in the stationary phase, enhanced the phosphorylation level of CovR in the rocA mutant. The phosphorylation levels of CovR in the CovS phosphatase-inactivated mutant and its rocA mutant were similar under acidic stress and Mg2+ (the signal that inhibits CovS phosphatase activity) treatments, suggesting that the phosphatase activity, but not the kinase activity, of CovS is required for RocA to modulate CovR phosphorylation. The phosphorylation level of CovR is crucial for GAS strains to regulate virulence factor expression; therefore, the growth phase- and pH-dependent RocA activity would contribute significantly to GAS pathogenesis.IMPORTANCE The emergence of invasive group A streptococcal infections has been reported worldwide. Clinical isolates that have spontaneous mutations or a truncated allele of the rocA gene (e.g., emm3-type isolates) are considered to be more virulent than isolates with the intact rocA gene (e.g., emm1-type isolates). RocA is a positive regulator of covR and has been shown to enhance the phosphorylation level of intracellular CovR regulator through the functional CovS protein. CovS is the membrane-embedded sensor and modulates the phosphorylation level of CovR by its kinase and phosphatase activities. The present study shows that the enhancement of CovR phosphorylation is mediated via the repression of CovS's phosphatase activity by RocA. In addition, we found that RocA acts dominantly on modulating CovR phosphorylation under neutral pH conditions and in the exponential phase of growth. The phosphorylation level of CovR is crucial for group A Streptococcus species to regulate virulence factor expression and is highly related to bacterial invasiveness; therefore, growth phase- and pH-dependent RocA activity and the sequence polymorphisms of rocA gene would contribute significantly to bacterial phenotype variations and pathogenesis.
Keywords: CovR-CovS; RocA; group A Streptococcus; pH.
Copyright © 2020 Chiang-Ni et al.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
