Opsonization-Enhanced Antigen Presentation by MR1 Activates Rapid Polyfunctional MAIT Cell Responses Acting as an Effector Arm of Humoral Antibacterial Immunity
- PMID: 32434941
- PMCID: PMC7311261
- DOI: 10.4049/jimmunol.2000003
Opsonization-Enhanced Antigen Presentation by MR1 Activates Rapid Polyfunctional MAIT Cell Responses Acting as an Effector Arm of Humoral Antibacterial Immunity
Abstract
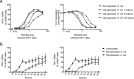
Mucosa-associated invariant T (MAIT) cells are innate-like antimicrobial T cells recognizing a breadth of important pathogens via presentation of microbial riboflavin metabolite Ags by MHC class Ib-related (MR1) molecules. However, the interaction of human MAIT cells with adaptive immune responses and the role they may play in settings of vaccinology remain relatively little explored. In this study we investigated the interplay between MAIT cell-mediated antibacterial effector functions and the humoral immune response. IgG opsonization of the model microbe Escherichia coli with pooled human sera markedly enhanced the capacity of monocytic APC to stimulate MAIT cells. This effect included greater sensitivity of recognition and faster response kinetics, as well as a markedly higher polyfunctionality and magnitude of MAIT cell responses involving a range of effector functions. The boost of MAIT cell responses was dependent on strongly enhanced MR1-mediated Ag presentation via increased FcγR-mediated uptake and signaling primarily mediated by FcγRI. To investigate possible translation of this effect to a vaccine setting, sera from human subjects before and after vaccination with the 13-valent-conjugated Streptococcus pneumoniae vaccine were assessed in a MAIT cell activation assay. Interestingly, vaccine-induced Abs enhanced Ag presentation to MAIT cells, resulting in more potent effector responses. These findings indicate that enhancement of Ag presentation by IgG opsonization allows innate-like MAIT cells to mount a faster, stronger, and qualitatively more complex response and to function as an effector arm of vaccine-induced humoral adaptive antibacterial immunity.
Copyright © 2020 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




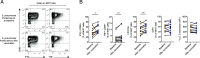
References
-
- Toubal A., Nel I., Lotersztajn S., Lehuen A. 2019. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 19: 643–657. - PubMed
-
- Gherardin N. A., McCluskey J., Rossjohn J., Godfrey D. I. 2018. The diverse family of MR1-restricted T cells. J. Immunol. 201: 2862–2871. - PubMed
-
- Provine N. M., Klenerman P. 2020. MAIT cells in health and disease. Annu. Rev. Immunol. 38: 203–228. - PubMed
-
- Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., Lantz O. 2011. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117: 1250–1259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

