Self-assembling influenza nanoparticle vaccines drive extended germinal center activity and memory B cell maturation
- PMID: 32434990
- PMCID: PMC7259527
- DOI: 10.1172/jci.insight.136653
Self-assembling influenza nanoparticle vaccines drive extended germinal center activity and memory B cell maturation
Abstract
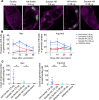
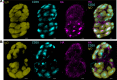
Protein-based, self-assembling nanoparticles elicit superior immunity compared with soluble protein vaccines, but the immune mechanisms underpinning this effect remain poorly defined. Here, we investigated the immunogenicity of a prototypic ferritin-based nanoparticle displaying influenza hemagglutinin (HA) in mice and macaques. Vaccination of mice with HA-ferritin nanoparticles elicited higher serum antibody titers and greater protection against experimental influenza challenge compared with soluble HA protein. Germinal centers in the draining lymph nodes were expanded and persistent following HA-ferritin vaccination, with greater deposition of antigen that colocalized with follicular dendritic cells. Our findings suggest that a highly ordered and repetitive antigen array may directly drive germinal centers through a B cell-intrinsic mechanism that does not rely on ferritin-specific T follicular helper cells. In contrast to mice, enhanced immunogenicity of HA-ferritin was not observed in pigtail macaques, where antibody titers and lymph node immunity were comparable to soluble vaccination. An improved understanding of factors that drive nanoparticle vaccine immunogenicity in small and large animal models will facilitate the clinical development of nanoparticle vaccines for broad and durable protection against diverse pathogens.
Keywords: Adaptive immunity; Immunology; Influenza; Nanotechnology; Vaccines.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

