Fatigue, quality of life and physical fitness following an exercise intervention in multiple myeloma survivors (MASCOT): an exploratory randomised Phase 2 trial utilising a modified Zelen design
- PMID: 32435057
- PMCID: PMC7374110
- DOI: 10.1038/s41416-020-0866-y
Fatigue, quality of life and physical fitness following an exercise intervention in multiple myeloma survivors (MASCOT): an exploratory randomised Phase 2 trial utilising a modified Zelen design
Abstract
Background: Exercise may improve fatigue in multiple myeloma survivors, but trial evidence is limited, and exercise may be perceived as risky in this older patient group with osteolytic bone destruction.
Methods: In this Phase 2 Zelen trial, multiple myeloma survivors who had completed treatment at least 6 weeks ago, or were on maintenance only, were enrolled in a cohort study and randomly assigned to usual care or a 6-month exercise programme of tailored aerobic and resistance training. Outcome assessors and usual care participants were masked. The primary outcome was the FACIT-F fatigue score with higher scores denoting less fatigue.
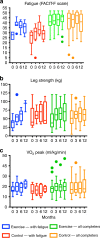
Results: During 2014-2016, 131 participants were randomised 3:1 to intervention (n = 89) or usual care (n = 42) to allow for patients declining allocation to the exercise arm. There was no difference between groups in fatigue at 3 months (between-group mean difference: 1.6 [95% CI: -1.1-4.3]) or 6 months (0.3 [95% CI: -2.6-3.1]). Muscle strength improved at 3 months (8.4 kg [95% CI: 0.5-16.3]) and 6 months (10.8 kg [95% CI: 1.2-20.5]). Using per-protocol analysis, cardiovascular fitness improved at 3 months (+1.2 ml/kg/min [95% CI: 0.3-3.7]). In participants with clinical fatigue (n = 17), there was a trend towards less fatigue with exercise over 6 months (6.3 [95% CI: -0.6-13.3]). There were no serious adverse events.
Conclusions: Exercise appeared safe and improved muscle strength and cardiovascular fitness, but benefits in fatigue appeared limited to participants with clinical fatigue at baseline. Future studies should focus on patients with clinical fatigue.
Clinical trial registration: The study was registered with ISRCTN (38480455) and is completed.
Conflict of interest statement
Professor Kwee Yong reports grants from Celgene during the conduct of the study. The remaining authors declare no competing interest.
Figures
Comment in
-
Rising to the challenge: designing, implementing and reporting exercise oncology trials in understudied populations.Br J Cancer. 2020 Jul;123(2):173-175. doi: 10.1038/s41416-020-0868-9. Epub 2020 May 21. Br J Cancer. 2020. PMID: 32435056 Free PMC article.
References
-
- O’Donnell EK, Raje NS. Myeloma bone disease: pathogenesis and treatment. Clin. Adv. Hematol. Oncol. 2017;15:285–295. - PubMed
-
- Shi Q, Wang XS, Shah N, Orlowski RZ, Qazilbash MH, Williams LA, et al. Prevalence of high symptom burden and its impact on functioning and quality of life in patients with multiple myeloma 3–9 months following autologous transplant. J. Clin. Oncol. 2014;32:e19580–e19580. doi: 10.1200/jco.2014.32.15_suppl.e19580. - DOI

