Targeting Plasmids to Limit Acquisition and Transmission of Antimicrobial Resistance
- PMID: 32435238
- PMCID: PMC7219019
- DOI: 10.3389/fmicb.2020.00761
Targeting Plasmids to Limit Acquisition and Transmission of Antimicrobial Resistance
Abstract
Antimicrobial resistance (AMR) is a significant global threat to both public health and the environment. The emergence and expansion of AMR is sustained by the enormous diversity and mobility of antimicrobial resistance genes (ARGs). Different mechanisms of horizontal gene transfer (HGT), including conjugation, transduction, and transformation, have facilitated the accumulation and dissemination of ARGs in Gram-negative and Gram-positive bacteria. This has resulted in the development of multidrug resistance in some bacteria. The most clinically significant ARGs are usually located on different mobile genetic elements (MGEs) that can move intracellularly (between the bacterial chromosome and plasmids) or intercellularly (within the same species or between different species or genera). Resistance plasmids play a central role both in HGT and as support elements for other MGEs, in which ARGs are assembled by transposition and recombination mechanisms. Considering the crucial role of MGEs in the acquisition and transmission of ARGs, a potential strategy to control AMR is to eliminate MGEs. This review discusses current progress on the development of chemical and biological approaches for the elimination of ARG carriers.
Keywords: CRISPR; antibiotics; infection; plasmid curing; resistance.
Copyright © 2020 Vrancianu, Popa, Bleotu and Chifiriuc.
Figures

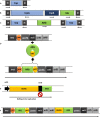

Similar articles
-
Pooled Plasmid Sequencing Reveals the Relationship Between Mobile Genetic Elements and Antimicrobial Resistance Genes in Clinically Isolated Klebsiella pneumoniae.Genomics Proteomics Bioinformatics. 2020 Oct;18(5):539-548. doi: 10.1016/j.gpb.2020.12.002. Epub 2020 Dec 30. Genomics Proteomics Bioinformatics. 2020. PMID: 33385612 Free PMC article.
-
Evolution of horizontal transmission in antimicrobial resistance plasmids.Microbiology (Reading). 2022 Jul;168(7). doi: 10.1099/mic.0.001214. Microbiology (Reading). 2022. PMID: 35849537 Review.
-
Importance of mobile genetic elements for dissemination of antimicrobial resistance in metagenomic sewage samples across the world.PLoS One. 2023 Oct 19;18(10):e0293169. doi: 10.1371/journal.pone.0293169. eCollection 2023. PLoS One. 2023. PMID: 37856515 Free PMC article.
-
In silico analyses of diversity and dissemination of antimicrobial resistance genes and mobile genetics elements, for plasmids of enteric pathogens.Front Microbiol. 2023 Jan 26;13:1095128. doi: 10.3389/fmicb.2022.1095128. eCollection 2022. Front Microbiol. 2023. PMID: 36777021 Free PMC article.
-
Microbial evolution through horizontal gene transfer by mobile genetic elements.Microb Biotechnol. 2024 Jan;17(1):e14408. doi: 10.1111/1751-7915.14408. Epub 2024 Jan 16. Microb Biotechnol. 2024. PMID: 38226780 Free PMC article. Review.
Cited by
-
Gene therapy to enhance angiogenesis in chronic wounds.Mol Ther Nucleic Acids. 2022 Aug 17;29:871-899. doi: 10.1016/j.omtn.2022.08.020. eCollection 2022 Sep 13. Mol Ther Nucleic Acids. 2022. PMID: 36159590 Free PMC article. Review.
-
Plasmid-encoded gene duplications of extended-spectrum β-lactamases in clinical bacterial isolates.Front Cell Infect Microbiol. 2024 Feb 26;14:1343858. doi: 10.3389/fcimb.2024.1343858. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38469349 Free PMC article.
-
Therapeutic effect of biosynthetic gold nanoparticles on multidrug-resistant Escherichia coli and Salmonella species isolated from ruminants.Vet World. 2021 Dec;14(12):3200-3210. doi: 10.14202/vetworld.2021.3200-3210. Epub 2021 Dec 29. Vet World. 2021. PMID: 35153413 Free PMC article.
-
Structural and functional diversity of type IV secretion systems.Nat Rev Microbiol. 2024 Mar;22(3):170-185. doi: 10.1038/s41579-023-00974-3. Epub 2023 Oct 9. Nat Rev Microbiol. 2024. PMID: 37814112 Free PMC article. Review.
-
Co-resistance and plasmid-mediated co-dissemination of florfenicol and azithromycin resistance in non-O157 Shiga toxin-producing Escherichia coli from cattle in Xinjiang, China.BMC Microbiol. 2025 Jul 30;25(1):465. doi: 10.1186/s12866-025-04172-4. BMC Microbiol. 2025. PMID: 40739480 Free PMC article.
References
-
- Adetosoye A. I., Rotilu I. O. (1985). Infectious drug resistance and antibiotic resistance curing in Salmonella and Shigella isolates from cases of diarrhoea. Revue d’elevage Med. Vet. Pays Trop. 38 433–437. - PubMed
-
- Antonoglou O., Lafazanis K., Mourdikoudis S., Vourlias G., Lialiaris T., Pantazaki A., et al. (2019). Biological relevance of CuFeO2 nanoparticles: antibacterial and anti-inflammatory activity, genotoxicity. DNA and protein interactions. Mater. Sci. Eng. C Mater. Biol. Appl. 99 264–274. 10.1016/j.msec.2019.01.112 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

