Comparative Analysis of Thymic and Blood Treg in Myasthenia Gravis: Thymic Epithelial Cells Contribute to Thymic Immunoregulatory Defects
- PMID: 32435245
- PMCID: PMC7218102
- DOI: 10.3389/fimmu.2020.00782
Comparative Analysis of Thymic and Blood Treg in Myasthenia Gravis: Thymic Epithelial Cells Contribute to Thymic Immunoregulatory Defects
Abstract
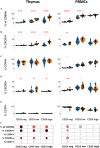
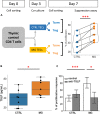
The thymus is involved in autoimmune Myasthenia gravis (MG) associated with anti-acetylcholine (AChR) antibodies. In MG, thymic regulatory T cells (Treg) are not efficiently suppressive, and conventional T cells (Tconv) are resistant to suppression. To better understand the specific role of the thymus in MG, we compared the phenotype and function of peripheral and thymic Treg and Tconv from controls and MG patients. Suppression assays with thymic or peripheral CD4 + T cells showed that the functional impairment in MG was more pronounced in the thymus than in the periphery. Phenotypic analysis of Treg showed a significant reduction of resting and effector Treg in the thymus but not in the periphery of MG patients. CD31, a marker lost with excessive immunoreactivity, was significantly reduced in thymic but not blood resting Treg. These results suggest that an altered thymic environment may explain Treg differences between MG patients and controls. Since thymic epithelial cells (TECs) play a major role in the generation of Treg, we co-cultured healthy thymic CD4 + T cells with control or MG TECs and tested their suppressive function. Co-culture with MG TECs consistently hampers regulatory activity, as compared with control TECs, suggesting that MG TECs contribute to the immune regulation defects of MG CD4 + T cells. MG TECs produced significantly higher thymic stromal lymphopoietin (TSLP) than control TECs, and a neutralizing anti-TSLP antibody partially restored the suppressive capacity of Treg derived from co-cultures with MG TECs, suggesting that TSLP contributed to the defect of thymic Treg in MG patients. Finally, a co-culture of MG CD4 + T cells with control TECs restored numbers and function of MG Treg, demonstrating that a favorable environment could correct the immune regulation defects of T cells in MG. Altogether, our data suggest that the severe defect of thymic Treg is at least partially due to MG TECs that overproduce TSLP. The Treg defects could be corrected by replacing dysfunctional TECs by healthy TECs. These findings highlight the role of the tissue environment on the immune regulation.
Keywords: CD31; PBMC; TSLP; Treg; immune regulation; myasthenia gravis; thymic epithelial cells; thymus.
Copyright © 2020 Truffault, Nazzal, Verdier, Gradolatto, Fadel, Roussin, Eymard, Le Panse and Berrih-Aknin.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

