HIVconsv Vaccines and Romidepsin in Early-Treated HIV-1-Infected Individuals: Safety, Immunogenicity and Effect on the Viral Reservoir (Study BCN02)
- PMID: 32435247
- PMCID: PMC7218169
- DOI: 10.3389/fimmu.2020.00823
HIVconsv Vaccines and Romidepsin in Early-Treated HIV-1-Infected Individuals: Safety, Immunogenicity and Effect on the Viral Reservoir (Study BCN02)
Abstract
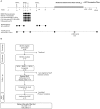
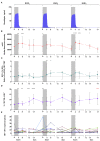
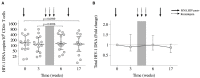
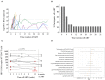
Kick&kill strategies combining drugs aiming to reactivate the viral reservoir with therapeutic vaccines to induce effective cytotoxic immune responses hold potential to achieve a functional cure for HIV-1 infection. Here, we report on an open-label, single-arm, phase I clinical trial, enrolling 15 early-treated HIV-1-infected individuals, testing the combination of the histone deacetylase inhibitor romidepsin as a latency-reversing agent and the MVA.HIVconsv vaccine. Romidepsin treatment resulted in increased histone acetylation, cell-associated HIV-1 RNA, and T-cell activation, which were associated with a marginally significant reduction of the viral reservoir. Vaccinations boosted robust and broad HIVconsv-specific T cells, which were strongly refocused toward conserved regions of the HIV-1 proteome. During a monitored ART interruption phase using plasma viral load over 2,000 copies/ml as a criterium for ART resumption, 23% of individuals showed sustained suppression of viremia up to 32 weeks without evidence for reseeding the viral reservoir. Results from this pilot study show that the combined kick&kill intervention was safe and suggest a role for this strategy in achieving an immune-driven durable viremic control.
Keywords: HDAC inhibitor; HIVconsv; early-treatment; kick&kill strategy; romidepsin.
Copyright © 2020 Mothe, Rosás-Umbert, Coll, Manzardo, Puertas, Morón-López, Llano, Miranda, Cedeño, López, Alarcón-Soto, Melis, Langohr, Barriocanal, Toro, Ruiz, Rovira, Carrillo, Meulbroek, Crook, Wee, Miró, Clotet, Valle, Martinez-Picado, Hanke, Brander, Moltó and the BCN02 Study Investigators.
Figures

References
-
- Davey RT, Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar RN, et al. . HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. (1999) 96:15109–14. 10.1073/pnas.96.26.15109 - DOI - PMC - PubMed
-
- Schooley RT, Spritzler J, Wang H, Lederman MM, Havlir D, Kuritzkes DR, et al. . AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1–infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. (2010) 202:705–16. 10.1086/655468 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

