5,6-Dihydroxypyrimidine Scaffold to Target HIV-1 Nucleocapsid Protein
- PMID: 32435383
- PMCID: PMC7236274
- DOI: 10.1021/acsmedchemlett.9b00608
5,6-Dihydroxypyrimidine Scaffold to Target HIV-1 Nucleocapsid Protein
Abstract
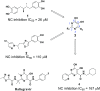
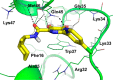
The HIV-1 nucleocapsid (NC) protein is a small basic DNA and RNA binding protein that is absolutely necessary for viral replication and thus represents a target of great interest to develop new anti-HIV agents. Moreover, the highly conserved sequence offers the opportunity to escape the drug resistance (DR) that emerged following the highly active antiretroviral therapy (HAART) treatment. On the basis of our previous research, nordihydroguaiaretic acid 1 acts as a NC inhibitor showing moderate antiviral activity and suboptimal drug-like properties due to the presence of the catechol moieties. A bioisosteric catechol replacement approach led us to identify the 5-dihydroxypyrimidine-6-carboxamide substructure as a privileged scaffold of a new class of HIV-1 NC inhibitors. Hit validation efforts led to the identification of optimized analogs, as represented by compound 28, showing improved NC inhibition and antiviral activity as well as good ADME and PK properties.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- UNAIDS . Global HIV & AIDS statistics - 2019 fact sheet; UNAIDS, 2020. https://www.unaids.org/en/resources/fact-sheet (accessed 2020-03-20).
-
- AIDSinfo . Understanding HIV/AIDS-Fact Sheets; AIDSinfo, 2020. https://aidsinfo.nih.gov/understanding-hiv-aids (accessed on 2020-03-20).
LinkOut - more resources
Full Text Sources
Chemical Information

