Synthesis and Antiproliferative Activity of Nitric Oxide-Donor Largazole Prodrugs
- PMID: 32435394
- PMCID: PMC7236235
- DOI: 10.1021/acsmedchemlett.9b00643
Synthesis and Antiproliferative Activity of Nitric Oxide-Donor Largazole Prodrugs
Abstract
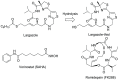
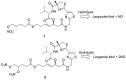
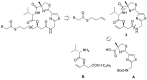
The marine natural product Largazole is the most potent Class I HDAC inhibitor identified to date. Since its discovery, many research groups have been attracted by the structural complexity and the peculiar anticancer activity, due to its capability to discriminate between tumor cells and normal cells. Herein, we discuss the synthesis and the in vitro biological profile of hybrid analogues of Largazole, as dual HDAC inhibitor and nitric oxide (NO) donors, potentially useful as anticancer agents. In particular, the metabolic stability of the modified thioester moiety of Largazole, bearing the NO-donor function/s, the in vitro release of NO, and the antiproliferative activity in tumor cell lines are presented.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
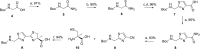
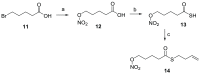
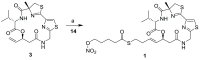

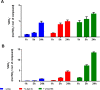
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

