Chiral Separation, X-ray Structure, and Biological Evaluation of a Potent and Reversible Dual Binding Site AChE Inhibitor
- PMID: 32435398
- PMCID: PMC7236231
- DOI: 10.1021/acsmedchemlett.9b00656
Chiral Separation, X-ray Structure, and Biological Evaluation of a Potent and Reversible Dual Binding Site AChE Inhibitor
Abstract
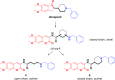
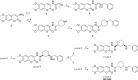
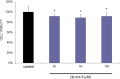
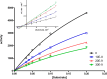
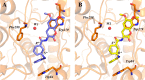
Acetylcholinesterase (AChE) inhibitors (AChEIs) still remain the leading therapeutic options for the symptomatic treatment of cognitive deficits associated with mild-to-moderate Alzheimer's disease. The search for new AChEIs benefits from well-established knowledge of the molecular interactions of selective AChEIs, such as donepezil and related dual binding site inhibitors. Starting from a previously disclosed coumarin-based inhibitor (±)-cis-1, active as racemate in the nanomolar range toward AChE, we proceeded on a double track by (i) achieving chiral resolution of the enantiomers of 1 by HPLC and (ii) preparing two close achiral analogues of 1, i.e., compounds 4 and 6. An eudismic ratio as high as 20 was observed for the (-) enantiomer of cis-1. The X-ray crystal structure of the complex between the (-)-cis-1 eutomer (coded as MC1420) and T. californica AChE was determined at 2.8 Å, and docking calculation results suggested that the eutomer in (1R,3S) absolute configuration should be energetically more favored in binding the enzyme than the eutomer in (1S,3R) configuration. The achiral analogues 4 and 6 were less effective in inhibiting AChE compared to (±)-cis-1, but interestingly butylamide 4 emerged as a potent inhibitor of butyrylcholinesterase (BChE).
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

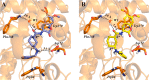
References
-
- 2019 Alzheimer’s disease facts and figures. Alzheimer's Dementia 2019, 15, 321–387. 10.1016/j.jalz.2019.01.010. - DOI
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

