Identification of 2-(4-(Phenylsulfonyl)piperazine-1-yl)pyrimidine Analogues as Novel Inhibitors of Chikungunya Virus
- PMID: 32435404
- PMCID: PMC7236252
- DOI: 10.1021/acsmedchemlett.9b00662
Identification of 2-(4-(Phenylsulfonyl)piperazine-1-yl)pyrimidine Analogues as Novel Inhibitors of Chikungunya Virus
Abstract
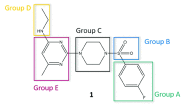
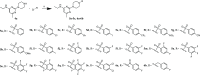
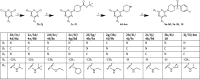
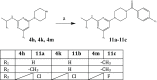
The chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus, and it is the causative agent of chikungunya fever (CHIKF). Although it has re-emerged as an epidemic threat, so far there are neither vaccines nor pharmacotherapy available to prevent or treat an infection. Herein, we describe the synthesis and structure-activity relationship studies of a class of novel small molecule inhibitors against CHIKV and the discovery of a new potent inhibitor (compound 6a). The starting point of the optimization process was N-ethyl-6-methyl-2-(4-(4-fluorophenylsulfonyl)piperazine-1-yl)pyrimidine-4-amine (1) with an EC50 of 8.68 μM, a CC50 of 122 μM, and therefore a resulting selectivity index (SI) of 14.2. The optimized compound 6a, however, displays a much lower micromolar antiviral activity (EC50 value of 3.95 μM), considerably better cytotoxic liability (CC50 value of 260 μM) and consequently an improved SI of greater than 61. Therefore, we report the identification of a promising novel compound class that has the potential for further development of antiviral drugs against the CHIKV.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
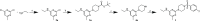

Similar articles
-
Discovery and development of labdane-oxindole hybrids as small-molecule inhibitors against chikungunya virus infection.Eur J Med Chem. 2022 Feb 15;230:114110. doi: 10.1016/j.ejmech.2022.114110. Epub 2022 Jan 14. Eur J Med Chem. 2022. PMID: 35085859
-
Trisubstituted Thieno[3,2-b]pyrrole 5-Carboxamides as Potent Inhibitors of Alphaviruses.J Med Chem. 2015 Dec 10;58(23):9196-213. doi: 10.1021/acs.jmedchem.5b01047. Epub 2015 Nov 19. J Med Chem. 2015. PMID: 26540338
-
Identification of [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones as novel inhibitors of Chikungunya virus replication.J Med Chem. 2014 May 22;57(10):4000-8. doi: 10.1021/jm401844c. Epub 2014 May 6. J Med Chem. 2014. PMID: 24800626
-
Computer-Aided Structure Based Drug Design Approaches for the Discovery of New Anti-CHIKV Agents.Curr Comput Aided Drug Des. 2017 Nov 10;13(4):346-361. doi: 10.2174/1573409913666170309145308. Curr Comput Aided Drug Des. 2017. PMID: 28294048 Review.
-
Recent Progress in Vaccine Development Against Chikungunya Virus.Front Microbiol. 2019 Dec 19;10:2881. doi: 10.3389/fmicb.2019.02881. eCollection 2019. Front Microbiol. 2019. PMID: 31921059 Free PMC article. Review.
Cited by
-
Advances in antiviral strategies targeting mosquito-borne viruses: cellular, viral, and immune-related approaches.Virol J. 2025 Feb 4;22(1):26. doi: 10.1186/s12985-025-02622-z. Virol J. 2025. PMID: 39905499 Free PMC article. Review.
-
Antiviral Strategies against Arthritogenic Alphaviruses.Microorganisms. 2020 Sep 7;8(9):1365. doi: 10.3390/microorganisms8091365. Microorganisms. 2020. PMID: 32906603 Free PMC article. Review.
-
Small-Molecule Inhibitors of Chikungunya Virus: Mechanisms of Action and Antiviral Drug Resistance.Antimicrob Agents Chemother. 2020 Nov 17;64(12):e01788-20. doi: 10.1128/AAC.01788-20. Print 2020 Nov 17. Antimicrob Agents Chemother. 2020. PMID: 32928738 Free PMC article. Review.
-
A review on structural genomics approach applied for drug discovery against three vector-borne viral diseases: Dengue, Chikungunya and Zika.Virus Genes. 2022 Jun;58(3):151-171. doi: 10.1007/s11262-022-01898-5. Epub 2022 Apr 8. Virus Genes. 2022. PMID: 35394596 Review.
-
Structural Insights into the Mechanisms of Action of Functionally Distinct Classes of Chikungunya Virus Nonstructural Protein 1 Inhibitors.Antimicrob Agents Chemother. 2021 Jun 17;65(7):e0256620. doi: 10.1128/AAC.02566-20. Epub 2021 Jun 17. Antimicrob Agents Chemother. 2021. PMID: 33875421 Free PMC article.
References
-
- Burt F. J.; Chen W.; Miner J. J.; Lenschow D. J.; Merits A.; Schnettler E.; Kohl A.; Rudd P. A.; Taylor A.; Herrero L. J.; et al. Chikungunya Virus: An Update on the Biology and Pathogenesis of This Emerging Pathogen. Lancet Infect. Dis. 2017, 17 (4), e107–e117. 10.1016/S1473-3099(16)30385-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

