Highly Promising Antitumor Agent of a Novel Platinum(II) Complex Bearing a Tetradentate Chelating Ligand
- PMID: 32435409
- PMCID: PMC7236228
- DOI: 10.1021/acsmedchemlett.9b00676
Highly Promising Antitumor Agent of a Novel Platinum(II) Complex Bearing a Tetradentate Chelating Ligand
Abstract
A new mononuclear cationic platinum(II) coordination compound with 6,6'-bis(NH-benzimidazol-2-yl)-2,2'-bipyridine (L) ligand having N4-tetradentate binding pocket [Pt(L)]Cl2·2H2O (Complex 1) was synthesized and characterized by FT-IR(ATR), UV-vis, 1H NMR, APCI and MALDI MS, and CHN analysis. The antigrowth effect of Complex 1 was tested in breast cancer (MDA-MB-231), lung cancer (A549), colorectal cancer (HCT-116), prostate cancer (PC-3) cell lines, and bronchial epithelial cell line (BEAS-2B) by the SRB and ATP cell viability assays. Apoptosis was detected with Annexin V, mitopotential, BCL-2 inactivation, and γH2AX assays by flow cytometry. Complex 1 was found to have cytotoxic activity of MDA-MB-231, A549, HCT-116, and PC-3 cancer cell lines in a dose-dependent manner for 48 h. Complex 1 has been found to cause cell death through different mechanisms depending on the type of cancer. The findings indicated that complex induced intrinsic apoptosis with the increased mitochondrial membrane depolarization level, Bcl-2 inactivation, and DNA damage in PC-3 and A549 cell lines.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

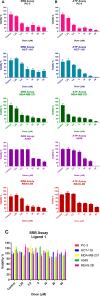
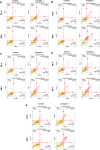

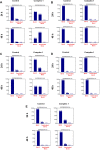

References
-
- Bloemink M. J.; Engelking H.; Karentzopoulos S.; Krebs B.; Reedijk J. Synthesis, Crystal Structure, Antitumor Activity, and DNA-Binding Properties of the New Active Platinum Compound (Bis (N-methylimidazol-2-yl) carbinol) dichloroplatinum (II), Lacking a NH Moiety, and of the Inactive Analog Dichloro (N 1, N 1 ‘-dimethyl-2, 2 ‘-biimidazole) platinum (II). Inorg. Chem. 1996, 35 (3), 619–627. 10.1021/ic950584j. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

