Hypersensitivity Reactions to Biologicals: from Bench to Bedside
- PMID: 32435575
- PMCID: PMC7222858
- DOI: 10.1007/s40521-020-00242-2
Hypersensitivity Reactions to Biologicals: from Bench to Bedside
Abstract
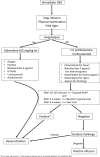
Purpose of review: Biologic agents are new treatment options for chronic inflammatory diseases and cancers. As a result of their unique mechanism of action, they are more effective and less toxic treatment option and their clinical usage is increasing. While they are more commonly used, various adverse effects have been observed including life-threatening ones, including anaphylaxis. The aim of this review is to distinguish the anaphylaxis from other hypersensitivity reactions (HSR) and provide a management algorithm for the anaphylactic reactions induced by biological agents.
Recent findings: Many case reports and series have been published regarding anaphylaxis and other hypersensitivity reactions (concerning cytokine release syndrome, acute infusion-related reactions) due to biologic agents. Although acute treatment of HSR varies according to the clinical presentation, desensitization with the drug is the major management option for subsequent administrations in the case of anaphylactic reactions.
Summary: Anaphylaxis and other immediate onset hypersensitivity reactions are occasionally difficult to differentiate from each other, and mixed-type reactions may be observed. Immediate management of anaphylaxis includes discontinuation of infusion, immediate administration of adrenaline, antihistamines, corticosteroids, and other treatment options depending on the symptoms. After 30-120 min of the reaction, a blood sample for serum tryptase levels should be obtained and after 4-6 weeks skin testing with the culprit drug should be performed for decision of long-term management via either graded challenge or desensitization.
Keywords: Anaphylaxis; Biologic agents; Hypersensitivity reactions; Monoclonal antibodies.
© Springer Nature Switzerland AG 2020.
Conflict of interest statement
Conflict of InterestAysegul Akarsu declares that he has no conflict of interest. Ozge Soyer declares that he has no conflict of interest. Bulent Enis Sekerel declares that he has no conflict of interest.
Figures
Similar articles
-
Biologics and anaphylaxis.Curr Opin Allergy Clin Immunol. 2019 Oct;19(5):439-446. doi: 10.1097/ACI.0000000000000550. Curr Opin Allergy Clin Immunol. 2019. PMID: 31169597 Review.
-
Diagnoses and Management of Drug Hypersensitivity and Anaphylaxis in Cancer and Chronic Inflammatory Diseases: Reactions to Taxanes and Monoclonal Antibodies.Clin Rev Allergy Immunol. 2018 Jun;54(3):375-385. doi: 10.1007/s12016-016-8556-5. Clin Rev Allergy Immunol. 2018. PMID: 27277133 Review.
-
Hypersensitivity reactions to biologics (part I): allergy as an important differential diagnosis in complex immune-derived adverse events.Allergo J Int. 2020;29(4):97-125. doi: 10.1007/s40629-020-00126-6. Epub 2020 May 12. Allergo J Int. 2020. PMID: 32421085 Free PMC article. Review.
-
Hypersensitivity to biological agents-updated diagnosis, management, and treatment.J Allergy Clin Immunol Pract. 2015 Mar-Apr;3(2):175-85; quiz 186. doi: 10.1016/j.jaip.2014.12.006. J Allergy Clin Immunol Pract. 2015. PMID: 25754718 Review.
-
Management of hypersensitivity reactions: a nursing perspective.Oncology (Williston Park). 2009 Feb;23(2 Suppl 1):26-30. Oncology (Williston Park). 2009. PMID: 19385164 Review.
Cited by
-
Temporal Modulation of Drug Desensitization Procedures.Curr Issues Mol Biol. 2022 Feb 8;44(2):833-844. doi: 10.3390/cimb44020057. Curr Issues Mol Biol. 2022. PMID: 35723342 Free PMC article. Review.
-
Atezolizumab-induced anaphylactic shock in a patient with hepatocellular carcinoma undergoing immunotherapy: A case report.World J Clin Cases. 2021 Jun 6;9(16):4110-4115. doi: 10.12998/wjcc.v9.i16.4110. World J Clin Cases. 2021. PMID: 34141773 Free PMC article.
-
The relationship between drug-induced immunogenicity and hypersensitivity reactions and skin tests related to infliximab, etanercept and adalimumab in patients with rheumatoid arthritis and ankylosing spondylitis.Turk J Med Sci. 2024 May 16;54(6):1310-1318. doi: 10.55730/1300-0144.5914. eCollection 2024. Turk J Med Sci. 2024. PMID: 39734351 Free PMC article.
-
Hypersensitivity reactions to multiple biologicals in a pediatric patient with severe persistent asthma.Proc (Bayl Univ Med Cent). 2022 Sep 1;36(1):66-67. doi: 10.1080/08998280.2022.2116692. eCollection 2023. Proc (Bayl Univ Med Cent). 2022. PMID: 36578600 Free PMC article.
-
Hypersensitivity and Immune-related Adverse Events in Biologic Therapy.Clin Rev Allergy Immunol. 2022 Jun;62(3):413-431. doi: 10.1007/s12016-021-08879-w. Epub 2021 Jul 28. Clin Rev Allergy Immunol. 2022. PMID: 34319562 Review.
References
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
-
- Li GN, Wang SP, Xue X, Qu XJ, Liu HP. Monoclonal antibody-related drugs for cancer therapy. Drug Discov Ther. 2013;7(5):178–184. - PubMed
-
- Approvals FaDAB, Year b. https://www.fda.gov/vaccines-blood-biologics/development-approval-proces.... Accessed 11/ 11 /2019.
-
- https://www.who.int/medicines/services/inn/Revised_mAb_nomenclature_sche.... Revised monoclonal antibody (mAb) nomenclature scheme. Geneva, 26 May 2017.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials