Hypersensitivity Reactions to Biologicals: from Bench to Bedside
- PMID: 32435575
- PMCID: PMC7222858
- DOI: 10.1007/s40521-020-00242-2
Hypersensitivity Reactions to Biologicals: from Bench to Bedside
Abstract
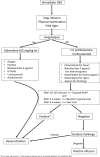
Purpose of review: Biologic agents are new treatment options for chronic inflammatory diseases and cancers. As a result of their unique mechanism of action, they are more effective and less toxic treatment option and their clinical usage is increasing. While they are more commonly used, various adverse effects have been observed including life-threatening ones, including anaphylaxis. The aim of this review is to distinguish the anaphylaxis from other hypersensitivity reactions (HSR) and provide a management algorithm for the anaphylactic reactions induced by biological agents.
Recent findings: Many case reports and series have been published regarding anaphylaxis and other hypersensitivity reactions (concerning cytokine release syndrome, acute infusion-related reactions) due to biologic agents. Although acute treatment of HSR varies according to the clinical presentation, desensitization with the drug is the major management option for subsequent administrations in the case of anaphylactic reactions.
Summary: Anaphylaxis and other immediate onset hypersensitivity reactions are occasionally difficult to differentiate from each other, and mixed-type reactions may be observed. Immediate management of anaphylaxis includes discontinuation of infusion, immediate administration of adrenaline, antihistamines, corticosteroids, and other treatment options depending on the symptoms. After 30-120 min of the reaction, a blood sample for serum tryptase levels should be obtained and after 4-6 weeks skin testing with the culprit drug should be performed for decision of long-term management via either graded challenge or desensitization.
Keywords: Anaphylaxis; Biologic agents; Hypersensitivity reactions; Monoclonal antibodies.
© Springer Nature Switzerland AG 2020.
Conflict of interest statement
Conflict of InterestAysegul Akarsu declares that he has no conflict of interest. Ozge Soyer declares that he has no conflict of interest. Bulent Enis Sekerel declares that he has no conflict of interest.
Figures
References
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
-
- Li GN, Wang SP, Xue X, Qu XJ, Liu HP. Monoclonal antibody-related drugs for cancer therapy. Drug Discov Ther. 2013;7(5):178–184. - PubMed
-
- Approvals FaDAB, Year b. https://www.fda.gov/vaccines-blood-biologics/development-approval-proces.... Accessed 11/ 11 /2019.
-
- https://www.who.int/medicines/services/inn/Revised_mAb_nomenclature_sche.... Revised monoclonal antibody (mAb) nomenclature scheme. Geneva, 26 May 2017.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials