Dopamine modulates individual differences in avoidance behavior: A pharmacological, immunohistochemical, neurochemical and volumetric investigation
- PMID: 32435668
- PMCID: PMC7231994
- DOI: 10.1016/j.ynstr.2020.100219
Dopamine modulates individual differences in avoidance behavior: A pharmacological, immunohistochemical, neurochemical and volumetric investigation
Abstract
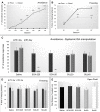
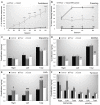
Avoidance behavior is a hallmark in pathological anxiety disorders and results in impairment of daily activities. Individual differences in avoidance responses are critical in determining vulnerability or resistance to anxiety disorders. Dopaminergic activation is implicated in the processing of avoidance responses; however, the mechanisms underlying these responses are unknown. In this sense, we used a preclinical model of avoidance behavior to investigate the possibility of an intrinsic differential dopaminergic pattern between good and poor performers. The specific goal was to assess the participation of dopamine (DA) through pharmacological manipulation, and we further evaluated the effects of systemic injections of the dopaminergic receptor type 1 (D1 antagonist - SCH23390) and dopaminergic receptor type 2 (D2 antagonist - sulpiride) antagonists in the good performers. Additionally, we evaluated the effects of intra-amygdala microinjection of a D1 antagonist (SCH23390) and a D2 antagonist (sulpiride) in good performers as well as intra-amygdala microinjection of a D1 agonist (SKF38393) and D2 agonist (quinpirole) in poor performers. Furthermore, we quantified the contents of dopamine and metabolites (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)) in the amygdala, evaluated the basal levels of tyrosine hydroxylase expression (catecholamine synthesis enzyme) and measured the volume of the substantia nigra, ventral tegmental area and locus coeruleus. Our results showed that it could be possible to convert animals from good to poor performers, and vice versa, by intra-amygdala (basolateral and central nucleus) injections of D1 receptor antagonists in good performers or D2 receptor agonists in poor performers. Additionally, the good performers had lower levels of DOPAC and HVA in the amygdala, an increase in the total volume of the amygdala (AMG), substantia nigra (SN), ventral tegmental area (VTA) and locus coeruleus (LC), and an increase in the number of tyrosine hydroxylase-positive cells in SN, VTA and LC, which positively correlates with the avoidance behavior. Taken together, our data show evidence for a dopaminergic signature of avoidance performers, emphasizing the role of distinct dopaminergic receptors in individual differences in avoidance behavior based on pharmacological, immunohistochemical, neurochemical and volumetric analyses. Our findings provide a better understanding of the role of the dopaminergic system in the execution of avoidance behavior.
Keywords: Amygdala; Anxiety disorders; Aversive learning; Avoidance; Dopamine; Prefrontal cortex.
© 2020 The Authors.
Conflict of interest statement
The authors report no conflicts of interest.
Figures






References
-
- Aguilar M.A., Marí-Sanmillán M.I., Morant-Deusa J.J., Miñarro J. Different inhibition of conditioned avoidance response by clozapine and DA D1 and D2 antagonists in male mice. Behav. Neurosci. 2000 Apr;114(2):389–400. - PubMed
-
- American Psychiatric Association . American Psychiatric Pub; 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5R)
-
- Antelman S.M., Caggiula A.R. Norepinephrine-dopamine interactions and behavior. Science. 1977 Feb 18;195(4279):646–653. - PubMed
-
- Bardeen J.R., Tull M.T., Stevens E.N., Gratz K.L. Exploring the relationship between positive and negative emotional avoidance and anxiety symptom severity: the moderating role of attentional control. J. Behav. Ther. Exp. Psychiatr. 2014 Sep;45(3):415–420. doi: 10.1016/j.jbtep.2014.04.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources

