Open-channel blocking action of volatile anaesthetics desflurane and sevoflurane on human voltage-gated Kv 1.5 channel
- PMID: 32436224
- PMCID: PMC7393063
- DOI: 10.1111/bph.15105
Open-channel blocking action of volatile anaesthetics desflurane and sevoflurane on human voltage-gated Kv 1.5 channel
Abstract
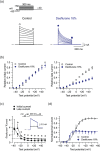
Background and purpose: Volatile anaesthetics have been shown to differentially modulate mammalian Shaker-related voltage-gated potassium (Kv 1.x) channels. This study was designed to investigate molecular and cellular mechanisms underlying the modulatory effects of desflurane or sevoflurane on human Kv 1.5 (hKv 1.5) channels.
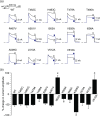
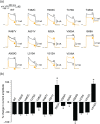
Experimental approach: Thirteen single-point mutations were constructed within pore domain of hKv 1.5 channel using site-directed mutagenesis. The effects of desflurane or sevoflurane on heterologously expressed wild-type and mutant hKv 1.5 channels were examined by whole-cell patch-clamp technique. A computer simulation was conducted to predict the docking pose of desflurane or sevoflurane within hKv 1.5 channel.
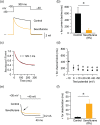
Key results: Both desflurane and sevoflurane increased hKv 1.5 current at mild depolarizations but decreased it at strong depolarizations, indicating that these anaesthetics produce both stimulatory and inhibitory actions on hKv 1.5 channels. The inhibitory effect of desflurane or sevoflurane on hKv 1.5 channels arose primarily from its open-channel blocking action. The inhibitory action of desflurane or sevoflurane on hKv 1.5 channels was significantly attenuated in T480A, V505A, and I508A mutant channels, compared with wild-type channel. Computational docking simulation predicted that desflurane or sevoflurane resides within the inner cavity of channel pore and has contact with Thr479, Thr480, Val505, and Ile508.
Conclusion and implications: Desflurane and sevoflurane exert an open-channel blocking action on hKv 1.5 channels by functionally interacting with specific amino acids located within the channel pore. This study thus identifies a novel molecular basis mediating inhibitory modulation of hKv 1.5 channels by desflurane and sevoflurane.
© 2020 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Putative binding sites for arachidonic acid on the human cardiac Kv 1.5 channel.Br J Pharmacol. 2015 Nov;172(22):5281-92. doi: 10.1111/bph.13314. Epub 2015 Oct 22. Br J Pharmacol. 2015. PMID: 26292661 Free PMC article.
-
Interactions of Propofol With Human Voltage-gated Kv1.5 Channel Determined by Docking Simulation and Mutagenesis Analyses.J Cardiovasc Pharmacol. 2018 Jan;71(1):10-18. doi: 10.1097/FJC.0000000000000538. J Cardiovasc Pharmacol. 2018. PMID: 29283926
-
Identification of Verapamil Binding Sites Within Human Kv1.5 Channel Using Mutagenesis and Docking Simulation.Cell Physiol Biochem. 2019;52(2):302-314. doi: 10.33594/000000022. Epub 2019 Feb 28. Cell Physiol Biochem. 2019. PMID: 30816676
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
[New inhalation anesthetics].Anaesthesist. 1996 Aug;45(8):674-93. doi: 10.1007/s001010050301. Anaesthesist. 1996. PMID: 8967581 Review. German.
Cited by
-
Update on the Mechanism and Treatment of Sevoflurane-Induced Postoperative Cognitive Dysfunction.Front Aging Neurosci. 2021 Jul 8;13:702231. doi: 10.3389/fnagi.2021.702231. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34305576 Free PMC article. Review.
-
Open channel block of Kv1.5 channels by HMQ1611.Front Pharmacol. 2022 Sep 16;13:965086. doi: 10.3389/fphar.2022.965086. eCollection 2022. Front Pharmacol. 2022. PMID: 36188606 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

