EAACI Allergen Immunotherapy User's Guide
- PMID: 32436290
- PMCID: PMC7317851
- DOI: 10.1111/pai.13189
EAACI Allergen Immunotherapy User's Guide
Abstract
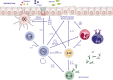
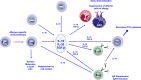
Allergen immunotherapy is a cornerstone in the treatment of allergic children. The clinical efficiency relies on a well-defined immunologic mechanism promoting regulatory T cells and downplaying the immune response induced by allergens. Clinical indications have been well documented for respiratory allergy in the presence of rhinitis and/or allergic asthma, to pollens and dust mites. Patients who have had an anaphylactic reaction to hymenoptera venom are also good candidates for allergen immunotherapy. Administration of allergen is currently mostly either by subcutaneous injections or by sublingual administration. Both methods have been extensively studied and have pros and cons. Specifically in children, the choice of the method of administration according to the patient's profile is important. Although allergen immunotherapy is widely used, there is a need for improvement. More particularly, biomarkers for prediction of the success of the treatments are needed. The strength and efficiency of the immune response may also be boosted by the use of better adjuvants. Finally, novel formulations might be more efficient and might improve the patient's adherence to the treatment. This user's guide reviews current knowledge and aims to provide clinical guidance to healthcare professionals taking care of children undergoing allergen immunotherapy.
Keywords: Allergy; immune regulation; immunotherapy; tolerance.
© 2020 The Authors. Pediatric Allergy and Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare financial support for several research grants, but no direct conflict of interest with this review article.
The authors declare that there is no conflict of interest in relation to this article.
P. M. Matricardi is a consultant for Hycor, Euroimmun, and Novartis; has received research funding from the Deutsche Forschungsgemeinschaft (DFG; grant no. MA‐4740/1‐1), Hycor Biomedical, and Euroimmun and reagents for research from Thermo Fisher; and receives speaker's fees from Euroimmun, Thermo Fisher Scientific, Stallergenes‐Greer, and HAL Allergy.
S. Halken reports personal fees and non‐financial support from ALK‐Abelló, outside the submitted work; G. Roberts has a patent issued: “Use of sublingual immunotherapy to prevent the development of allergy in at risk infants”; and his university has received payments for the activities he has undertaken giving expert advice to ALK, and presenting at company symposia for ALK, Allergen Therapeutics, and Meda, and serving as a member of an Independent Data Monitoring Committee for Merck outside of this work; Dr. Pfaar reports grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from ASIT Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from MEDA Pharma/MYLAN, grants and personal fees from Anergis S.A., personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Indoor Biotechnologies, grants from Glaxo Smith Kline, personal fees from Astellas Pharma Global, outside the submitted work. E. Angier reports being Secretary of Primary Care Interest Group EAACI. ALK conference SOSA meeting 2015. Previous paid advisory board one each for MEDA 2012, Stallergenes, 2012, Schering Plough 2009, and one paid lecture by MEDA; S. Arasi has nothing to disclose; A. Muraro reports speaker's fees from Aimmune DVB, Mylan, Nestlè outside the submitted work.
Oliver Pfaar reports grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from ASIT Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from Novartis Pharma, personal fees from MEDA Pharma, grants and personal fees from Anergis S.A., personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Pohl‐Boskamp, personal fees from Indoor Biotechnologies, grants from Glaxo Smith Kline, and personal fees from Astellas Pharma Global, outside the submitted work. Graham Roberts's University has received fees from ALK‐Abello and AllergoPharma for consultancy work he has undertaken outside of this work. Oliver Pfaar, Susanne Halken, Antonella Muraro, and Graham Roberts are authors of the EAACI Rhinoconjunctivitis AIT guideline.
P. Rodriguez del Rio received research funding from the Health Research Fund of Carlos III Health Institute, Foundation for Biomedical Research of the Niño Jesus University Children's Hospital, and Spanish Society of Allergology and Clinical Immunology Foundation and reports honoraria for consultancy and/or advisory board and/or lectures from ALK‐Abello, FAES Pharma, LETI Pharma, Merck, Aimmune, Allergy Therapeutics, MEDA Pharma, and Novartis. R. Bazire has nothing to disclose in relation to this article.
D. Barber declares to receive scientific consultancy fees from ALK‐Abello A/S and Aimmune Therapeutics; M Alvaro Lozano declares that there is no conflict of interest in relation to this article.
Authors declare no conflict of interest with regard to this article.
The authors declare there is no conflict of interest in relation to this article.
G. Sturm reports grants from ALK Abello, personal fees from Novartis , personal fees from Bencard, personal fees from Stallergens, personal fees from HAL, personal fees from Allergopharma, personal fees from Mylan, outside the submitted work. L. Arzt‐Gradwohl has nothing to disclose in relation to this article.
The authors declare that there is no conflict of interest in relation to this article.
Antonella Muraro reports speaker's fee for ALK, Stallergenes, Mylan, Nestlè. Susanne Halken was member of Steering Committee for the Grazax Asthma Prevention (GAP) study, published 2017; paid by ALK‐Abelló for participation in meetings only; since 2018 country coordinator (DK) and involved in a multicenter RCT (MT‐11) on HDM SLIT (Acarizax) for HDM allergic asthma in children; only study expenses were paid by the company.
Oliver Pfaar reports grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from ASIT Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from MEDA Pharma/MYLAN, grants and personal fees from Anergis S.A., personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Indoor Biotechnologies, grants from Glaxo Smith Kline, personal fees from Astellas Pharma Global, outside the submitted work.
Other authors have no conflict of interest to declare.
Dr. Shamji reports grants and personal fees from ASIT Biotech.sa, grants from ALK, grants from Regeneron, grants from Merck, grants from Immune Tolerance Network, grants from ASIT Biotech.sa, personal fees from ALK, personal fees from Allergopharma, outside the submitted work. The rest of the authors declare that there is no conflict of interest in relation to this article.
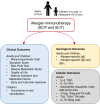
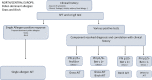
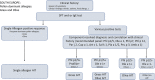
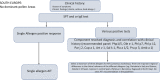
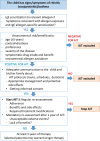
Taken from EAACI allergic rhinoconjunctivitis allergen immunotherapy guidelines. Short‐term benefit refers to when on immunotherapy while long‐term benefit refers to effect after immunotherapy stopped.
Taken from EAACI allergic rhinoconjunctivitis allergen immunotherapy guidelines. Short‐term benefit refers to when on immunotherapy while long‐term benefit refers to effect after immunotherapy stopped.
Figures










References
-
- Pajno GB, Castagnoli R, Antonella M, et al. Allergen Immunotherapy for IgE‐Mediated Food Allergy: there is a measure in everything to a proper proportion of therapy. Pediatr Allergy Immunol 2019;30(4):415‐422. - PubMed
-
- Pajno GB, Fernandez‐Rivas M, Arasi S, et al. EAACI Guidelines on allergen immunotherapy: IgE‐mediated food allergy. Allergy. 2018;73(4):799‐815. - PubMed
-
- Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy. 2018;73(4):765‐798. - PubMed
-
- Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE‐mediated food allergy: a systematic review and meta‐analysis. Allergy. 2017;72(8):1133‐1147. - PubMed
-
- Rigbi NE, Goldberg MR, Levy MB, Nachshon L, Golobov K, Elizur A. Changes in patient quality of life during oral immunotherapy for food allergy. Allergy. 2017;72(12):1883‐1890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

