Ultra rapid lispro lowers postprandial glucose and more closely matches normal physiological glucose response compared to other rapid insulin analogues: A phase 1 randomized, crossover study
- PMID: 32436641
- PMCID: PMC7540588
- DOI: 10.1111/dom.14094
Ultra rapid lispro lowers postprandial glucose and more closely matches normal physiological glucose response compared to other rapid insulin analogues: A phase 1 randomized, crossover study
Abstract
Aims: To compare the pharmacokinetic (PK) and glucodynamic (GD) characteristics of ultra rapid lispro (URLi; Eli Lilly and Company, Indianapolis, Indiana), Fiasp® (Novo Nordisk, Bagsvaerd, Denmark), Humalog® (Eli Lilly and Company) and NovoRapid® (Novo Nordisk), in patients with type 1 diabetes (T1D).
Materials and methods: This was a randomized, double-blind, four-period, crossover study, conducted in 68 patients with T1D. Patients received the same individualized subcutaneous dose of each study drug immediately prior to a liquid test meal. For comparison, 12 healthy subjects received the same test meal.
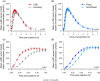
Results: URLi had a significantly faster insulin absorption compared to the other insulins tested. Early half-maximal drug concentration was reached 13 minutes after administration of URLi, which was 6 minutes faster than Fiasp, 13 minutes faster than Humalog, and 14 minutes faster than NovoRapid (all P <0.0001). Early insulin exposure was significantly greater and late insulin exposure was reduced after URLi compared to the other insulins. URLi achieved the greatest numerical reduction in postprandial glucose (PPG) at 2 hours post-meal (7 mg/dL vs Fiasp) and was significantly different from Humalog (21 mg/dL) and Novo Rapid (29 mg/dL). Additionally, glucose excursions over the first 3 hours post-meal with URLi were comparable to those in healthy subjects.
Conclusions: URLi demonstrated the fastest insulin absorption and the greatest numeric PPG-lowering effect compared to the other insulins tested. URLi more closely matched the early physiological glucose control observed in healthy subjects.
Keywords: glycaemic control; insulin analogues; pharmacodynamics; pharmacokinetics; randomized trial; type 1 diabetes.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
H.L., D.E.C., E.L., J.B.‐V., Q.Z., M.A.D. and J.L. are employees and shareholders of Eli Lilly and Company. T.H., E.Z. and C.K. are employees of Profil. T.H. and C.K. are also shareholders of Profil. T.H. received speaker honoraria and travel grants from Eli Lilly and Company.
Figures




References
-
- Pratt E. Treprostinil causes local vasodilation, is well tolerated, and results in faster absorption of insulin Lispro. Diabetes. 2017;66(Supplement 1):975.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

