PLA2G1B is involved in CD4 anergy and CD4 lymphopenia in HIV-infected patients
- PMID: 32436864
- PMCID: PMC7301387
- DOI: 10.1172/JCI131842
PLA2G1B is involved in CD4 anergy and CD4 lymphopenia in HIV-infected patients
Abstract
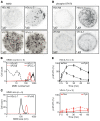
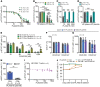
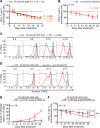
The precise mechanism leading to profound immunodeficiency of HIV-infected patients is still only partially understood. Here, we show that more than 80% of CD4+ T cells from HIV-infected patients have morphological abnormalities. Their membranes exhibited numerous large abnormal membrane microdomains (aMMDs), which trap and inactivate physiological receptors, such as that for IL-7. In patient plasma, we identified phospholipase A2 group IB (PLA2G1B) as the key molecule responsible for the formation of aMMDs. At physiological concentrations, PLA2G1B synergized with the HIV gp41 envelope protein, which appears to be a driver that targets PLA2G1B to the CD4+ T cell surface. The PLA2G1B/gp41 pair induced CD4+ T cell unresponsiveness (anergy). At high concentrations in vitro, PLA2G1B acted alone, independently of gp41, and inhibited the IL-2, IL-4, and IL-7 responses, as well as TCR-mediated activation and proliferation, of CD4+ T cells. PLA2G1B also decreased CD4+ T cell survival in vitro, likely playing a role in CD4 lymphopenia in conjunction with its induced IL-7 receptor defects. The effects on CD4+ T cell anergy could be blocked by a PLA2G1B-specific neutralizing mAb in vitro and in vivo. The PLA2G1B/gp41 pair constitutes what we believe is a new mechanism of immune dysfunction and a compelling target for boosting immune responses in HIV-infected patients.
Keywords: AIDS/HIV; Cytokines; Immunology; Lipid rafts; T cells.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

