Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review
- PMID: 32436994
- PMCID: PMC7280615
- DOI: 10.1002/jmv.26038
Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review
Abstract
Background: Current evidence suggests an important role of the interleukin-6 (IL-6) pathway in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related cytokine release storm in severely ill coronavirus disease 2019 (COVID-19) patients. Inhibition of the IL-6 pathway with tocilizumab has been employed successfully in some of these patients but the data is mostly consistent of case reports and series.
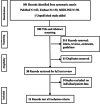
Methods: We performed a systematic search of PubMed, Embase, and Medline from 22nd April 2020 and again on 27th April 2020 using the following search terms alone or in combination: "COVID-19," "coronavirus," "SARS-CoV-2," "COVID," "anti-interleukin-6 receptor antibodies," "anti-IL-6," "tocilizumab," "sarilumab," "siltuximab." We included studies that reported individual patient data. We extracted and analyzed individual level data on baseline characteristics, laboratory findings, and clinical outcomes. The primary endpoint was in-hospital mortality. Secondary endpoints included in-hospital complications, recovery rates, effect of patient characteristics on the primary outcome and changes in levels of inflammatory markers.
Results: Three hundred fifty-two records were identified through a systematic search, of which 10 studies met the inclusion criteria. A single study currently under review was also added. Eleven observational studies encompassing 29 patients were included in the present review. There were more males (24 [82.8%]), and hypertension was the most common comorbidity (16 [48.3%]). Over an average of 5.4 hospital days, the primary endpoint occurred in 6 (20.7%) patients. Among surviving patients, about 10% had worsened disease and 17% recovered. The most common complication was acute respiratory distress syndrome (8 [27.6%]). The IL-6 level was significantly higher after the initiation of tocilizumab with median (interquartile range) of 376.6 (148-900.6) pg/mL compared to the baseline of 71.1 (31.9-122.8) pg/mL (P = .002). Mean (standard deviation) levels of C-reactive protein (CRP) were significantly decreased following treatment 24.6 (26.9) mg/L compared to baseline 140.4 (77) mg/L (P < .0001). Baseline demographics were not significantly different among survivors and nonsurvivors by Fisher's exact test.
Conclusion: In COVID-19 patients treated with tocilizumab, IL-6 levels are significantly elevated, which are supportive of cytokine storm. Following initiation of tocilizumab, there is elevation in the IL-6 levels and CRP levels dramatically decrease, suggesting an improvement in this hyperinflammatory state. Ongoing randomized control trials will allow for further evaluation of this promising therapy.
Importance: Recent data indicate that severe COVID-19 causes a cytokine release storm and is associated with worse clinical outcomes and IL-6 plays an important role. It is suggestive that anti-IL-6 results in the improvement of this hyperinflammatory state. However, to our knowledge, there is no individual patient data systematic review performed to summarize baseline characteristics and clinical outcomes of COVID-19 patients who received tocilizumab.
Keywords: COVID-19; SARS-CoV-2; anti-interleukin-6 receptor antibody; individual patient data; tocilizumab.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
References
-
- WHO. WHO Director‐General's opening remarks at the media briefing on COVID‐19—11 March 2020. https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐re...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous